3D print molecular models
3D printable molecular models
W. Tandy Grubbs, Professor & Chair, Department of Chemistry & Biochemistry24 July 2017
UniversityScience
Tandy Grubbs, Professor of Chemistry, writes about how chemistry students at Stetson University are taking advantage of 3D printing to better visualize the molecular world.
How can 3D printing be utilized to better understand chemistry? The concept of creating molecular models to serve as instructional aids has certainly been around a long time. Students of organic chemistry are often encouraged to purchase molecular modeling kits that can be used to build geometric renderings of various chemical structures. However, traditional ball-and-stick kits are limited with regard to how accurately the components can be pieced together to depict the true geometric form of a molecule. Perhaps there is a better way.
Low-cost 3D printing represents a powerful new tool that can be used by science educators and their students to create realistic, tangible models of chemical structures. 3D-printed molecular models are capable of doing far more than illustrate the atoms and bonds that make up a molecule. The models can often depict subtleties about the chemical structure that are difficult to discern using traditional modeling kits. 3D-printed models can even inform us about chemical reaction pathways—illustrating, for example, how different chemical entities fit together and interact in a three-dimensional fashion.
Students in the Department of Chemistry and Biochemistry at Stetson University, in collaboration with chemistry faculty mentors, have made use of a combination of software tools, web-resources, and 3D printing to create several different types of chemical models. Simple ball-and-stick models of common chemical structures have been constructed. More realistic, space-filling models of organic compounds, crystal structures, proteins, and other molecular complexes have also been fabricated. Originally, 3D-printed molecular models were created by students as part of independent study and senior research experiences. More recently, instructors have incorporated 3D printing activities as part of the required chemistry curriculum. 3D printing activities of this type can often make difficult to learn chemistry concepts more accessible to students and can greatly enhanced enthusiasm and motivation for learning abstract material.
More recently, instructors have incorporated 3D printing activities as part of the required chemistry curriculum. 3D printing activities of this type can often make difficult to learn chemistry concepts more accessible to students and can greatly enhanced enthusiasm and motivation for learning abstract material.
‘Drug Design’: 3D-printed molecular models were constructed that illustrate the subtle structural differences between two common topical anti-inflammatory drugs, hydrocortisone (left) and desonide (right).
Each student from the Stetson Physical Chemistry class completed a lab activity whereby a unique molecule was selected and computational chemistry software was used to determine the optimized geometry of the molecule. Students then used 3D printing to create a physical model of the resulting structure.
A molecular model of the compound isopropyl alcohol; C3H7OH (aka rubbing alcohol) is 3D-printed on an Ultimaker 2+ Extended printer, using the Ultimaker Cura software.
Are you are a student who is interested in creating your own 3D printable chemical models? Or perhaps you are an educator who hopes to get students involved in similar activities. In either case, you are encouraged to check out the step-by-step tutorial (that can be accessed at the link shown immediately below) which utilizes a free, open source molecular editor and visualization tool called ‘Avogadro’ to draw a molecule from scratch. A second free software tool called the ‘Python Molecular Viewer’ is subsequently used to convert the ‘Avogadro’ chemical model into an STL file that can be 3D-printed.
Check out the 3D Printable Molecular Model lesson
ACKNOWLEDGEMENT:
We wish to thank Betty Drees Johnson for her ongoing financial support of the Stetson University duPont-Ball Library Innovation Lab.
Molecular models for biochemistry, biotechnology to biomedical science
Based in part on Wrapp et al Science 2020. The University of Texas at Austin
Molecular model showing structure of the 2019-nCoV Spike Glycoprotein with ACE2
Based in part on Wrapp et al Science 2020. The University of Texas at Austin
The University of Texas at Austin
Molecular model showing structure of the 2019-nCoV Spike Glycoprotein with ACE2
Based in part on Wrapp et al Science 2020. The University of Texas at Austin
Molecular model showing structure of the 2019-nCoV Spike Glycoprotein with ACE2
Molecular Models in collaboration with Lee 3D, have been working with life-science researchers and scientists across the UK and beyond to bring molecular structures to life using colour 3D printing. We printed the SARS-Cov-2 spike trimer for Prof. Jason McLellan (University of Texas at Austin).
Copies of the model have been gifted to the vaccine development teams at Oxford University and Imperial College London. These models are hugely beneficial as communication aids in public outreach.
The SARS-CoV-2 spike protein is a key target for a vaccine against Covid-19. It is termed a ‘spike’ protein since dozens of copies of this molecule sit on the outside of each tiny corona virus; these give each virus a distinct spiky appearance – like a crown (or corona).
The spike protein is made of three protein chains (shown in red, green and blue on the model) that wind together to form a trimer. When a Covid-19 virus infects a human, the tip of each spike can interact with a human protein that is naturally present in the cell membrane of our respiratory epithelia – angiotensin-converting enzyme (ACE2).
The ACE2 molecule is shown in yellow and the magnets show how it can specifically attach to the spike trimer. When the spike binds to ACE2, this can lead to the virus binding and then infecting the human cell. The trimer is also covered in small sugar molecules called glycans and the biological function of these and their role in infection is still being studied.
The structure we have printed comes from cryo-EM data published by the group of Prof. Jason McLellan, University of Texas at Austin, in the journal Science in February 2020:
Wrapp et al, (2020), Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation, Science, 367, 1260–1263
Jiang et al, (2016), Structures of a CRISPR-Cas9 R-loop complex primed for DNA cleavage, Science, 351, 867–871
Molecular Model showing structure of the CRISPR-Cas9 complex
Jiang et al, (2016), Structures of a CRISPR-Cas9 R-loop complex primed for DNA cleavage, Science, 351, 867–871
Molecular Model showing structure of the CRISPR-Cas9 complex
Jiang et al, (2016), Structures of a CRISPR-Cas9 R-loop complex primed for DNA cleavage, Science, 351, 867–871
Molecular Model showing structure of the CRISPR-Cas9 complex
This model, solved by Prof. Jennifer Doudna’s team at the University of California, Berkeley, shows the crystal structure of catalytically-active Streptococcus pyogenes CRISPR-Cas9 in complex with a single-guide RNA and a double-stranded viral DNA target. The complex is primed for DNA cleavage and shows how bacteria use a CRISPR-Cas nuclease to recognise and then degrade invading viral DNA – a form of bacterial immune system. This recognition is achieved with extraordinary specificity using a guide-RNA strand that directs the Cas9 enzyme to a specific DNA sequence. This property of being able to target, cut or change any DNA sequence with exquisite precision has led to the exciting and rapidly-expanding field of CRISPR-Cas gene-editing technologies.
Jennifer Doudna’s team at the University of California, Berkeley, shows the crystal structure of catalytically-active Streptococcus pyogenes CRISPR-Cas9 in complex with a single-guide RNA and a double-stranded viral DNA target. The complex is primed for DNA cleavage and shows how bacteria use a CRISPR-Cas nuclease to recognise and then degrade invading viral DNA – a form of bacterial immune system. This recognition is achieved with extraordinary specificity using a guide-RNA strand that directs the Cas9 enzyme to a specific DNA sequence. This property of being able to target, cut or change any DNA sequence with exquisite precision has led to the exciting and rapidly-expanding field of CRISPR-Cas gene-editing technologies.
The colourful hands-on model we have made is ideal for use in undergraduate and postgraduate lectures and practicals, allowing students to explore, measure and appreciate the close link between molecular structure and function. The model works well in any life science degree where gene-editing is taught: from genetics to biochemistry, biotechnology to biomedical science. The model is equally useful to researchers as a visual reference and hands-on demo in science communication and school/college outreach events.
The model is equally useful to researchers as a visual reference and hands-on demo in science communication and school/college outreach events.
The 25 cm-long model sits on a mounting stand, from which it can be easily removed. We have used ten different colours to identify ten key features/domains of the complex. This visual key is to aid learning: the guide-RNA is in red, the DNA strands are in light and dark blue and the Cas9 enzyme domains are in seven further colours. We provide a list of each feature – and what it does – with the model. We have added optional labels (that are attached to the structure) to help students visually identify the various parts of the structure.
As the photos above show, there are three magnetically-detachable sections that help reveal where the separated DNA strands and guide-RNA are; how the nuclease active sites are positioned next to the DNA, and how Cas9 encircles the nucleic acids.
The structure we have printed comes from X-ray crystallography data (PDB code 5F9R) published by the group of Prof. Jennifer Doudna, UC-Berkeley, in the journal Science in February 2016:
Jennifer Doudna, UC-Berkeley, in the journal Science in February 2016:
Jiang et al, (2016), Structures of a CRISPR-Cas9 R-loop complex primed for DNA cleavage, Science, 351, 867–871
This molecular model was a collaboration with Prof. Thomas Meier at Imperial College London. It shows all twenty-six subunits that comprise a fully functioning plant ATP synthase. ATP synthases – found across all three kingdoms of life – act like molecular turbines and generate large numbers of high-energy ATP molecules for cell use. The machine operates by directing protons (H+ ions) from one side of a membrane (here, the chloroplast thylakoid membrane) to the other.
The model is coloured by subunit to aid understanding of how the motor works. It comprises two major complexes, a water soluble F1 complex and the membrane-embedded (Fo) complex. Functionally, the ATP synthase can be divided in a stationary part (called the stator) and rotary part (called the rotor). Protons enter the Fo motor by the stator a-subunit (pale blue+red), which directs them onto the c-ring rotor (wheat). On each of the 14 c-ring subunits is a glutamate residue (red spot) that conducts the protons round almost 360° to an exit point on the a-subunit. The continuous movement of this proton stream (shown in red) is converted into rotary motion. The c-ring is directly attached to a rotor shaft that comprises the epsilon (magenta), and gamma subunits (blue+yellow). So as the c-ring rotates, the attached shaft spins at the same rate.
Protons enter the Fo motor by the stator a-subunit (pale blue+red), which directs them onto the c-ring rotor (wheat). On each of the 14 c-ring subunits is a glutamate residue (red spot) that conducts the protons round almost 360° to an exit point on the a-subunit. The continuous movement of this proton stream (shown in red) is converted into rotary motion. The c-ring is directly attached to a rotor shaft that comprises the epsilon (magenta), and gamma subunits (blue+yellow). So as the c-ring rotates, the attached shaft spins at the same rate.
The key feature of the model is that the Fo motor is free to rotate, providing a clear visual demonstration of how the top of the gamma subunit rotates deep within the stationary F1 head. The hexameric head is made from alternating alpha and beta subunits (dark and light green) and is held in place by the peripheral stalk, a scaffold of subunits made from the b-subunit (purple), b’-subunit (pink) and delta-subunit (orange). The ATP synthase rotation mechanism is fully reversible and can operate either in ATP synthesis mode (making ATP, using the proton-motive force), or in ATPase (hydrolysis) mode, to pump protons across the membranes and maintain the proton-motive force, depending on cell physiological conditions. The movie shows the ATP synthase operating in clockwise direction (when viewed from to of F1), which is in ATPase proton pumping direction.
The movie shows the ATP synthase operating in clockwise direction (when viewed from to of F1), which is in ATPase proton pumping direction.
The 20 cm-high model sits on a removable transparent frame, which fits into a base section. Using glossy colours to identify each of the 26 subunits and how they are positioned in relation to each other was a key part of the project brief. Also visible inside the alpha and beta headgroup are the ATP and ADP molecules, where ATP synthesis is occurring by the classic 3-stage binding-change mechanism.
The structure we have printed comes from cryo-EM data (PDB code 6FKF) and was published in the journal Science by the team of Alexander Hahn, Janet Vonck, Deryck Mills, Thomas Meier and Werner Kühlbrandt:
Hahn et al, (2018), Structure, mechanism and regulation of the chloroplast ATP synthase, Science, 360, eaat4318.
Structure of the F1Fo ATP Synthase
Structure of the F1Fo ATP Synthase
Structure of the F1Fo ATP Synthase
Structure of the F1Fo ATP Synthase
Structure of the F1Fo ATP Synthase
Structure of the F1Fo ATP Synthase
Oxyhaemoglobin
Breathe in! Human oxyhaemoglobin. This glossy model shows the four subunits of haemoglobin.
This glossy model shows the four subunits of haemoglobin.
Oxyhaemoglobin
Four subunits of haemoglobin. The two alpha subunits are in white and black; the two beta subunits are in light and medium grey.
Oxyhaemoglobin
The haem group in each monomer subunit is in red and shows where an O2 molecule binds – four dioxygens per haemoglobin tetramer.
Oxyhaemoglobin
Cut-away model through the middle of haemoglobin to reveal the complex polypeptide chain folding inside the molecule.
COX-2 with aspirin molecules
How aspirin works: structure of the enzyme cyclooxygenase 2 (COX-2). COX-2, is a homodimer and in this model the subunits are coloured white and blue; the haem group in each subunit required for prostaglandin synthesis is shown in red.
COX-2 with aspirin molecules
COX-2, also known as prostaglandin h3 synthase, is inhibited by the anti-inflammatory drug aspirin. Aspirin molecules are shown to scale alongside. Model made for the Biochemical Society outreach team.
C.Csp231I
This 2 part magnetic model shows how the complex assembles.
C.Csp231I
Structure of the Restriction-Modification controller protein C.Csp231I.
C.Csp231I
This protein consists of two dimers (orange/yellow and light/dark blue) that interact to hold the DNA in a loop.
Nucleosome Fibre
Six models of the 50 nm nucleosome fibre. Each model represents a different theoretical inter-nucleosomal spacing; nucleosomes are shown as yellow and orange spheres.
Nucleosome Fibre
These images shows how useful physical models can be for inspecting and evaluating molecular structures.
Nucleosome Fibre
These images shows how useful physical models can be for inspecting and evaluating molecular structures.
Researchers have discovered a way to print at the molecular level
Archive
Subscribe to the author
Subscribe
Don't want
It seems to us that someday, one fine day, nanofactories will appear on the planet, where things will be printed from molecules. We will turn garbage into whatever we want with the help of a powerful computer system that can break any object into molecules, and then reassemble them into something completely different.
We will turn garbage into whatever we want with the help of a powerful computer system that can break any object into molecules, and then reassemble them into something completely different.
And now we've heard rumors that a pair of researchers - Justin Baron, assistant professor of bioengineering at the Polytechnic University, and Ryan Zenger - have taken the first steps in this direction.
They combined the idea of 3D printing with molecular self-assembly and came up with "3D genetic printing" technology. For the benefit of non-biologists, molecular self-assembly is the process by which molecules take up a particular position without the involvement of any outside source. Molecular self-assembly is a bottom-up manufacturing method, just like 3D printing.
Thanks to an accidental discovery, researchers have managed to create proteins that can be converted into fibers. They were just trying to create a gluten binder from gluten proteins. What happened next shocked them. When they removed some of the protein, the fibers filled the tube on their own.
When they removed some of the protein, the fibers filled the tube on their own.
“I knew immediately that we had made a mistake,” recalls Baron. – I asked the student to put on a hairnet, cleaned the entire laboratory, sinning for dust and dirt. We started the experiments again and this time behaved much more accurately. But everything happened again. Then we started studying molecules and realized that we had stumbled upon something interesting.”
Researcher Justin Baron
The quality of the fibers resembled spider silk threads, which researchers have long and unsuccessfully tried to obtain. Spider silk has a high specific strength, five times that of steel. This is an excellent material for use in a variety of industries.
Scientists have realized that they can control the protein structures of the fibers and change their color. But that's not all. By combining gluten proteins with other proteins, they were able to print proteins from molecules, while endowing them with different properties. Usually, during printing, users use a special program to translate computer code and raw materials into a physical object. Here, the researchers found that they could use the genetic code as a computer code and obtain DNA by reverse counting, adding it to the host bacterium (in this case, E. coli). When the protein (raw material) grows, it leaves the cell and begins to interact with other proteins, forming fibers, the properties of which are predetermined by the researchers.
Usually, during printing, users use a special program to translate computer code and raw materials into a physical object. Here, the researchers found that they could use the genetic code as a computer code and obtain DNA by reverse counting, adding it to the host bacterium (in this case, E. coli). When the protein (raw material) grows, it leaves the cell and begins to interact with other proteins, forming fibers, the properties of which are predetermined by the researchers.
Baron and Zenger hope that they will eventually be able to use this method to produce various objects at the molecular level. Since protein fibers are a natural building material, you need to be prepared for anything. One day people will be able to create coffee pots, human bones and even muscles using this 3D printing method.
Now scientists continue to work on their discovery and produce silk-like fibers for further research. They are also looking for a way to increase the size of the fibers in order to be able to create larger objects.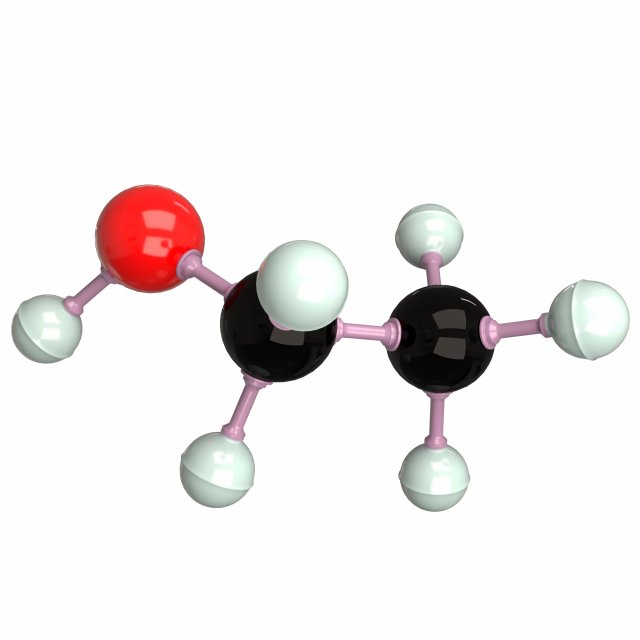
Article prepared for 3DToday.ru
Subscribe to the author
Subscribe
Don't want
Even more interesting articles
7
Subscribe to the author
Subscribe
Don't want
So, you are thinking about the possibility of making money on 3D printing. How realistic is this? Quite, although the way...
Read more
5
Subscribe to the author
Subscribe to the author
Don't want to
Alexander Gessler wrote a small handy program to convert 40+ 3D file formats (OBJ, S...
Read more
3
Subscribe to the author
Subscribe to the author
Don't want to
More and more RepRap 3D printers based on modeling technology appear on the market today. 0001
0001
New Molecular 3D Printing Technologies
As 3D printing of continues to advance biomedical engineering, a team of researchers from Queen Mary University of London (QMUL) is already preparing to take the next step. Lead researcher Alvaro Mata, Professor of Engineering and Materials Science at QMUL, along with co-author and PhD student Gastón Primo, are working hard to develop a new type of 3D printing specifically designed to recreate complex biological environments.
3DEAL is a state-of-the-art bioconversion technology refers to 3D electrophoresis lithography, a novel fabrication method capable of generating complex molecular structures in soft matter such as hydrogels. By giving scientists complete spatial control over the chemistry of the engineered environment, 3DEAL opens up a whole new way to recreate natural environments found in the human body, such as 3D molecular gradients or patterns. Combined with being a relatively simple and inexpensive manufacturing technology, 3DEAL is particularly promising due to its microscale resolution capabilities, which can be as deep as a few centimeters. A new type of bioengineering could be put to work that could improve drug screening platforms or build complex tissue constructs.
A new type of bioengineering could be put to work that could improve drug screening platforms or build complex tissue constructs.
As Mata explains, “The human body is primarily composed of anisotropic, hierarchical and mostly three-dimensional structures. New ways of making media that can recreate the physical and chemical properties of such structures will have important implications for the way more effective drugs are designed, or more functional tissues and organ structures can be engineered.”
Maintaining physical and chemical control of these recreated structures is a particularly interesting prospect and a major selling point of the team's new technology. One of the key design features of 3DEAL is the incorporation of an electric field and a porous mask that, when used together, allow researchers to navigate and localize on tees of different types of molecules within hydrogel structures. When it comes to 3DEAL, microscale resolution is possible as it operates at extremely high volumes.