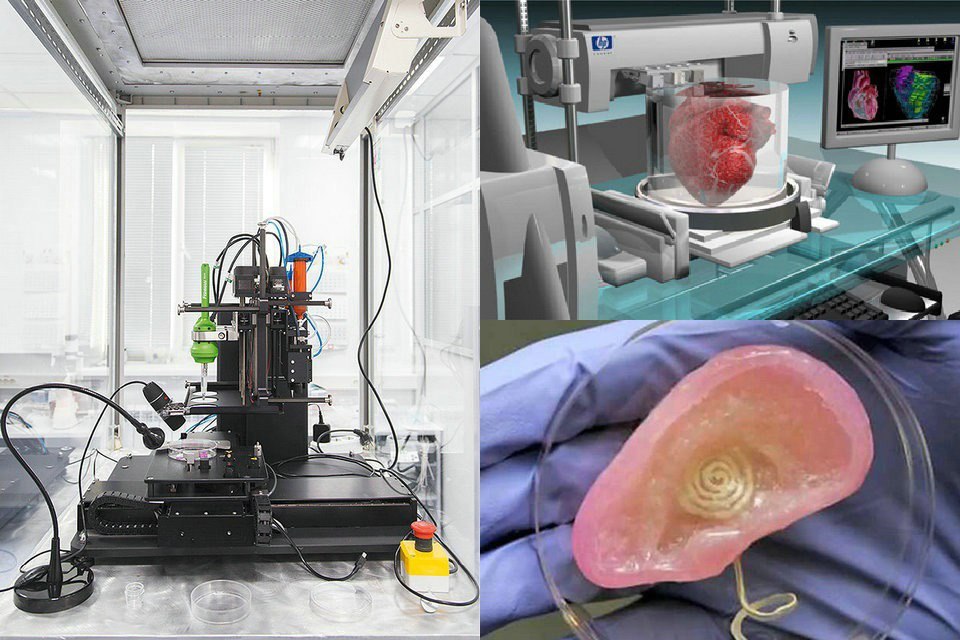
3D print stem cells
3D printed tissues and organs without the scaffolding -- ScienceDaily
Engineered tissues and organs have been grown with various degrees of success in labs for many years. Many of them have used a scaffolding approach where cells are seeded onto biodegradable supportive structures that provide the underlying architecture of the organ or tissue desired.
But scaffolds can be problematic -- ultimately, they should degrade and disappear, but timing that decomposition to coincide with the maturation of the organ is tricky, and sometimes degradation byproducts can be toxic. Scaffolds also can interfere with the development of cell-to-cell connections, which are important for the formation of functional tissues.
Now, a research team led by Eben Alsberg, the Richard and Loan Hill Professor of Bioengineering and Orthopaedics at the University of Illinois at Chicago, has developed a process that enables 3D printing of biological tissues without scaffolds using "ink" made up of only stem cells. They report their results in the journal Materials Horizons.
"Our cell only printing platform allows for the 3D printing of cells without a classical scaffold support using a temporary hydrogel bead bath in which printing takes place," Alsberg said.
The micron-scale hydrogel beads allow the nozzle of the 3D printer to move through it and deposit cells with minimal resistance to that nozzle movement or the ejection of the cells. The gel beads support the cells as they are printed and keeps them in place and preserves their shape.
Once the cells are printed into the hydrogel bead matrix, it is exposed to UV light, which cross-links the beads together, in effect freezing them in place. This lets the printed cells connect with each other, mature and grow within a stable structure. The media that bathes the cells flows easily through the cross-linked gel beads and can be changed out as needed to provide fresh nutrients and dispose of waste products made by the cells.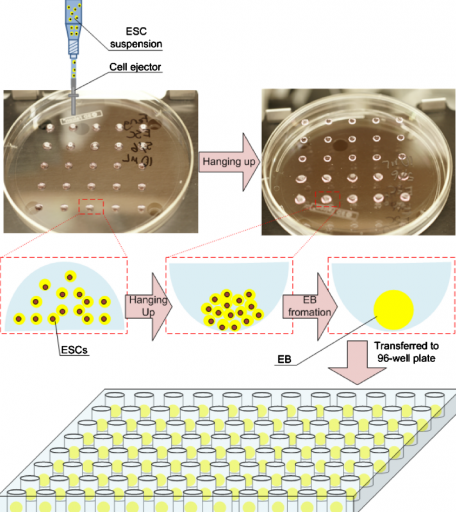 The hydrogel beads can be removed through gentle agitation, or controlling their degradation, leaving the intact tissue behind.
The hydrogel beads can be removed through gentle agitation, or controlling their degradation, leaving the intact tissue behind.
"The hydrogel bead bath has unique properties which allow for both printing of the cell-only bioink in complex architectures, and subsequent temporary stabilization of these cell-only structures to allow for cell-cell junctions to form," Alsberg said. "Using chemistry we can then regulate when the beads go away."
The cells Alsberg's team used are stem cells -- those that can differentiate into a wide variety of other cell types. They used the stem cells to 3D print a cartilage ear and a rodent-sized "femur" in the hydrogel bead bath. The cells they printed were able to form stable, cell-cell connections through specialized proteins.
"For the first time, cell-only constructs can be printed in intricate forms that are made up of different cell types without a hydrogel carrier or traditional scaffold that can then be stabilized for a period of a day to weeks. We've demonstrated that cell aggregates can be organized and assembled using this strategy to form larger functional tissues, which may be valuable for tissue engineering or regenerative medicine, drug screening and as models to study developmental biology," Alsberg said.
We've demonstrated that cell aggregates can be organized and assembled using this strategy to form larger functional tissues, which may be valuable for tissue engineering or regenerative medicine, drug screening and as models to study developmental biology," Alsberg said.
Story Source:
Materials provided by University of Illinois at Chicago. Note: Content may be edited for style and length.
3D bioprinting using stem cells
Review
. 2018 Jan;83(1-2):223-231.
doi: 10.1038/pr.2017.252. Epub 2017 Nov 1.
Chin Siang Ong 1 , Pooja Yesantharao 1 , Chen Yu Huang 2 , Gunnar Mattson 1 , Joseph Boktor 1 , Takuma Fukunishi 1 , Huaitao Zhang 1 , Narutoshi Hibino 1
Affiliations
Affiliations
- 1 Division of Cardiac Surgery, Johns Hopkins Hospital, Baltimore, MD.
- 2 Division of Cardiology, Johns Hopkins Hospital, Baltimore, MD.
- PMID: 28985202
- DOI: 10.1038/pr.2017.252
Review
Chin Siang Ong et al. Pediatr Res. 2018 Jan.
. 2018 Jan;83(1-2):223-231.
doi: 10.1038/pr.2017.252. Epub 2017 Nov 1.
Authors
Chin Siang Ong 1 , Pooja Yesantharao 1 , Chen Yu Huang 2 , Gunnar Mattson 1 , Joseph Boktor 1 , Takuma Fukunishi 1 , Huaitao Zhang 1 , Narutoshi Hibino 1
Affiliations
- 1 Division of Cardiac Surgery, Johns Hopkins Hospital, Baltimore, MD.
- 2 Division of Cardiology, Johns Hopkins Hospital, Baltimore, MD.
- PMID: 28985202
- DOI: 10.1038/pr.2017.252
Abstract
Recent advances have allowed for three-dimensional (3D) printing technologies to be applied to biocompatible materials, cells and supporting components, creating a field of 3D bioprinting that holds great promise for artificial organ printing and regenerative medicine. At the same time, stem cells, such as human induced pluripotent stem cells, have driven a paradigm shift in tissue regeneration and the modeling of human disease, and represent an unlimited cell source for tissue regeneration and the study of human disease. The ability to reprogram patient-specific cells holds the promise of an enhanced understanding of disease mechanisms and phenotypic variability. 3D bioprinting has been successfully performed using multiple stem cell types of different lineages and potency. The type of 3D bioprinting employed ranged from microextrusion bioprinting, inkjet bioprinting, laser-assisted bioprinting, to newer technologies such as scaffold-free spheroid-based bioprinting. This review discusses the current advances, applications, limitations and future of 3D bioprinting using stem cells, by organ systems.
The ability to reprogram patient-specific cells holds the promise of an enhanced understanding of disease mechanisms and phenotypic variability. 3D bioprinting has been successfully performed using multiple stem cell types of different lineages and potency. The type of 3D bioprinting employed ranged from microextrusion bioprinting, inkjet bioprinting, laser-assisted bioprinting, to newer technologies such as scaffold-free spheroid-based bioprinting. This review discusses the current advances, applications, limitations and future of 3D bioprinting using stem cells, by organ systems.
Similar articles
-
Advancing Frontiers in Bone Bioprinting.
Ashammakhi N, Hasan A, Kaarela O, Byambaa B, Sheikhi A, Gaharwar AK, Khademhosseini A. Ashammakhi N, et al. Adv Healthc Mater. 2019 Apr;8(7):e1801048. doi: 10.1002/adhm.201801048. Epub 2019 Feb 8. Adv Healthc Mater.
 2019. PMID: 30734530 Review.
2019. PMID: 30734530 Review. -
Three-dimensional bioprinting of stem-cell derived tissues for human regenerative medicine.
Skeldon G, Lucendo-Villarin B, Shu W. Skeldon G, et al. Philos Trans R Soc Lond B Biol Sci. 2018 Jul 5;373(1750):20170224. doi: 10.1098/rstb.2017.0224. Philos Trans R Soc Lond B Biol Sci. 2018. PMID: 29786559 Free PMC article. Review.
-
Recent advances in bioprinting techniques: approaches, applications and future prospects.
Li J, Chen M, Fan X, Zhou H. Li J, et al. J Transl Med. 2016 Sep 20;14:271. doi: 10.1186/s12967-016-1028-0. J Transl Med. 2016. PMID: 27645770 Free PMC article. Review.
-
Tissue Engineering Applications of Three-Dimensional Bioprinting.

Zhang X, Zhang Y. Zhang X, et al. Cell Biochem Biophys. 2015 Jul;72(3):777-82. doi: 10.1007/s12013-015-0531-x. Cell Biochem Biophys. 2015. PMID: 25663505 Review.
-
Three-dimensional bioprinting in tissue engineering and regenerative medicine.
Gao G, Cui X. Gao G, et al. Biotechnol Lett. 2016 Feb;38(2):203-11. doi: 10.1007/s10529-015-1975-1. Epub 2015 Oct 14. Biotechnol Lett. 2016. PMID: 26466597 Review.
See all similar articles
Cited by
-
Establishing a reproducible approach to study cellular functions of plant cells with 3D bioprinting.
Van den Broeck L, Schwartz MF, Krishnamoorthy S, Tahir MA, Spurney RJ, Madison I, Melvin C, Gobble M, Nguyen T, Peters R, Hunt A, Muhammad A, Li B, Stuiver M, Horn T, Sozzani R.
 Van den Broeck L, et al. Sci Adv. 2022 Oct 14;8(41):eabp9906. doi: 10.1126/sciadv.abp9906. Epub 2022 Oct 14. Sci Adv. 2022. PMID: 36240264 Free PMC article.
Van den Broeck L, et al. Sci Adv. 2022 Oct 14;8(41):eabp9906. doi: 10.1126/sciadv.abp9906. Epub 2022 Oct 14. Sci Adv. 2022. PMID: 36240264 Free PMC article. -
3D-printed microrobots from design to translation.
Dabbagh SR, Sarabi MR, Birtek MT, Seyfi S, Sitti M, Tasoglu S. Dabbagh SR, et al. Nat Commun. 2022 Oct 5;13(1):5875. doi: 10.1038/s41467-022-33409-3. Nat Commun. 2022. PMID: 36198675 Free PMC article. Review.
-
Skeletal Muscle Pathogenesis in Polyglutamine Diseases.
Marchioretti C, Zuccaro E, Pandey UB, Rosati J, Basso M, Pennuto M. Marchioretti C, et al. Cells. 2022 Jul 3;11(13):2105. doi: 10.3390/cells11132105. Cells. 2022. PMID: 35805189 Free PMC article. Review.
-
Current methods for fabricating 3D cardiac engineered constructs.

Rogozinski N, Yanez A, Bhoi R, Lee MY, Yang H. Rogozinski N, et al. iScience. 2022 Apr 29;25(5):104330. doi: 10.1016/j.isci.2022.104330. eCollection 2022 May 20. iScience. 2022. PMID: 35602954 Free PMC article. Review.
-
The 3D Bioprinted Scaffolds for Wound Healing.
Antezana PE, Municoy S, Álvarez-Echazú MI, Santo-Orihuela PL, Catalano PN, Al-Tel TH, Kadumudi FB, Dolatshahi-Pirouz A, Orive G, Desimone MF. Antezana PE, et al. Pharmaceutics. 2022 Feb 21;14(2):464. doi: 10.3390/pharmaceutics14020464. Pharmaceutics. 2022. PMID: 35214197 Free PMC article. Review.
See all "Cited by" articles
References
-
- Exp Cell Res. 2010 Apr 15;316(7):1159-68 - PubMed
-
- Acta Biomater.
 2016 Mar 1;32:170-177 - PubMed
2016 Mar 1;32:170-177 - PubMed
- Acta Biomater.
-
- Annu Rev Biomed Eng. 2014 Jul 11;16:247-76 - PubMed
-
- Adv Healthc Mater. 2013 Mar;2(3):442-9 - PubMed
-
- J Am Coll Cardiol. 2010 Aug 3;56(6):510-20 - PubMed
Publication types
MeSH terms
Substances
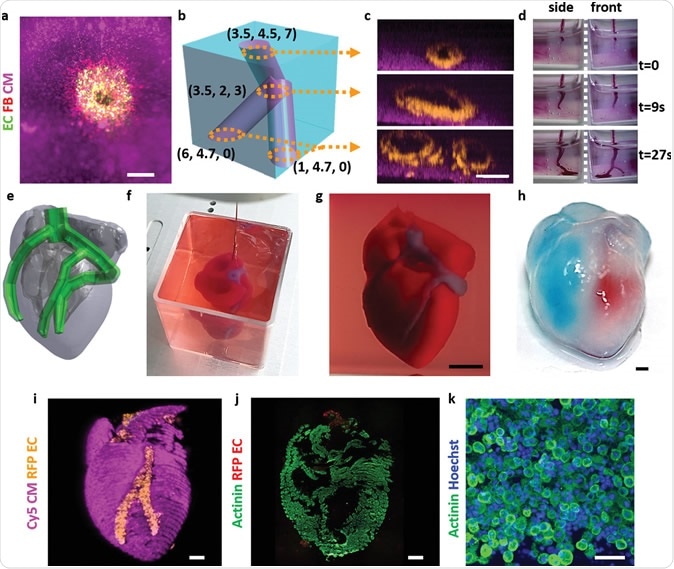
Stem cells self-organized and became a material for 3D bioprinting
Scientists have developed a method for printing living tissues on a 3D bioprinter that uses stem cells and one of their most important properties is self-organization. As reported in Nature Materials , stem cells from various tissues placed in favorable conditions self-organized and formed tissues that looked and functioned like full-fledged living tissues.
As reported in Nature Materials , stem cells from various tissues placed in favorable conditions self-organized and formed tissues that looked and functioned like full-fledged living tissues.
The formation of tissues in a living organism depends on intercellular contacts and the microenvironment of cells. In the process of development and life activity, cells form around themselves an extracellular matrix - a part of the tissue that serves as a mechanical support and information intermediary for cells. Cells are located in the matrix (respectively, in the tissue) in a spatial relationship characteristic of each organ. To be in the right place at the right time, cells express hundreds of receptors and chemicals that determine how the cell interacts with neighboring cells and the matrix. Thanks to such interactions, cells self-organize - each cell knows where it needs to be in the tissue and what it needs to do.
Until recently, scientists were unable to grow large organelles (more than a centimeter) using 3D bioprinting, either because the cells were too tightly attached to the environment and could not move, or the environment itself did not allow creating the necessary microenvironment.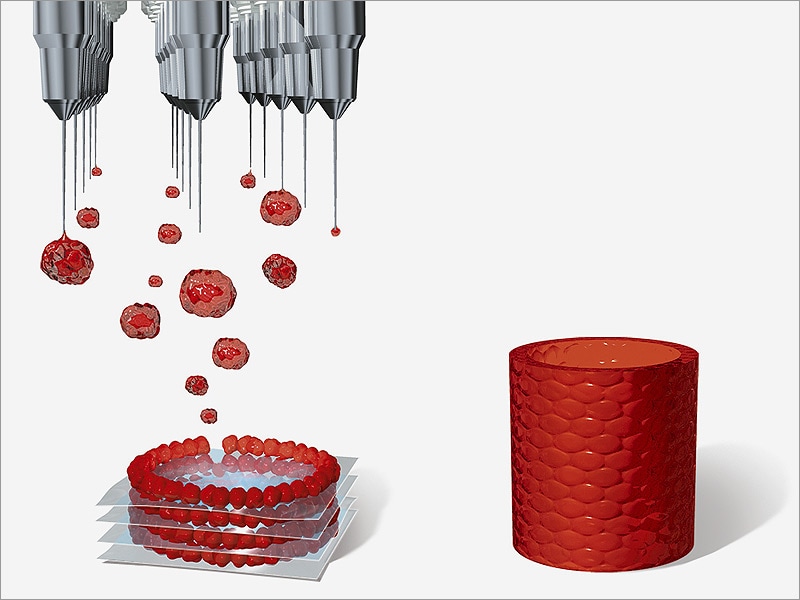 However, Matthias P. Lutolf and colleagues at the Federal Polytechnic School of Lausanne have developed a new 3D bioprinting approach that can solve these problems. The new method, which, among other advantages, allows microscopic work with cell mass and direct observation of the process of printing and growing, uses the self-organization of stem cells as the basis for growing full-fledged organs and tissues. This approach allows you to repeat the natural processes of development of tissues and organs.
However, Matthias P. Lutolf and colleagues at the Federal Polytechnic School of Lausanne have developed a new 3D bioprinting approach that can solve these problems. The new method, which, among other advantages, allows microscopic work with cell mass and direct observation of the process of printing and growing, uses the self-organization of stem cells as the basis for growing full-fledged organs and tissues. This approach allows you to repeat the natural processes of development of tissues and organs.
To demonstrate the potential and versatility of the new method, the scientists used human small intestine stem cells. The line-printed stem cells were placed on a nutrient medium made of hydrogel with collagen, which is similar in properties to the extracellular matrix. In this environment, cells easily moved and created a fibrous connective tissue structure around themselves, additionally turning the environment into a favorable microenvironment.
A few days later, the cells transformed into a whole and organized epithelial tube 5 to 15 millimeters long, surrounded by a specific matrix, in which scientists found the tissue organization found in the classical organelles of the small intestine.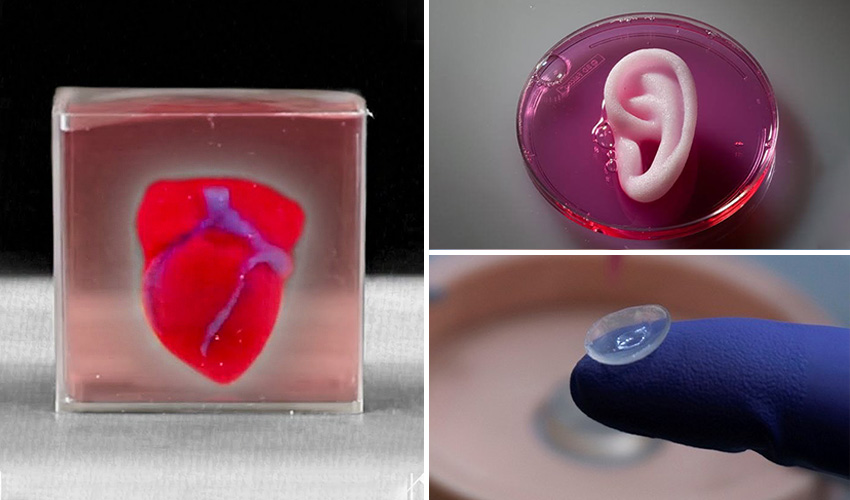 At the same time, scientists note that it was the nutrient medium and the extracellular matrix that had a great influence on the formation of the intestinal tube, which created a favorable microenvironment for cell self-organization. It is noteworthy that intercellular self-organization leveled small printing defects (for example, cell adhesion).
At the same time, scientists note that it was the nutrient medium and the extracellular matrix that had a great influence on the formation of the intestinal tube, which created a favorable microenvironment for cell self-organization. It is noteworthy that intercellular self-organization leveled small printing defects (for example, cell adhesion).
The scientists also managed to grow the epithelium of the mouse small intestine. At first, the stem cells were arranged in a line, but after four or six days, thanks to the self-organization of the cells, a gap appeared in this line, which turned it into a hollow tube. After another one or two days, crypts and villi characteristic of the epithelium of the small intestine were found in the tube, in which scientists found mature differentiated enterocytes, Paneth cells (protective cells that are found only in the small intestine), goblet and enteroendocrine cells. The entire tube responded to external stimuli — Paneth cells released bactericidal granules in response to chemical stimulation, and all cells swelled under the action of forskolin . These reactions show that the new bioprinting technique can produce engineered tissues with physiological responses reminiscent of those in living organisms.
The entire tube responded to external stimuli — Paneth cells released bactericidal granules in response to chemical stimulation, and all cells swelled under the action of forskolin . These reactions show that the new bioprinting technique can produce engineered tissues with physiological responses reminiscent of those in living organisms.
In addition, endothelial cells printed on a mixture with vascular endothelial growth factor (VEGF) formed capillary vessels de novo . Due to favorable conditions (VEGF, loose medium), the formation of capillaries was triggered at the tissue scale, which led to the formation of a vasculature with a continuous lumen.
All of these experiments show that the specific local interactions that govern the self-organization of a small cell block can extend to the tissue level and form different types of tissues: both endothelial and tissues of the internal environment. Using the property of self-organization of stem cells should be an important step towards growing tissues and organs in vitro , because in this case it will be possible to obtain functionally complete organs that can be used for transplantation or drug testing.
Using the property of self-organization of stem cells should be an important step towards growing tissues and organs in vitro , because in this case it will be possible to obtain functionally complete organs that can be used for transplantation or drug testing.
Recently, we told that another group of scientists from the same Swiss school has developed a 3D bioprinting method that differs from traditional layer-by-layer 3D printing in that the organ model is created simultaneously over the entire volume in one step, which reduces printing time to several tens of seconds.
Vyacheslav Gomenyuk
Found a typo? Select the fragment and press Ctrl+Enter.
3D printing of heart muscle cells / Sudo Null IT News
Heart cells under a microscope.
The dream of 3D printing heart tissue is one step closer, thanks to the development of scientists from the Heart Research Institute (HRI), Sydney, Australia.
Article by Sophie Scott from abc.net.au translated for you by Top 3D Shop .
Key points:
- Scientists hope artificial tissues can replace those damaged by heart attacks;
- Cells behave like real ones, they beat and move;
- Researchers hope the technology will be available to patients within the next five years.
Scientists are using a new bioprinter to print cells they say could replace a patient's damaged heart cells.
“The process will go something like this: when a patient enters the clinic, a tissue sample is taken from him, namely the skin, from which we extract cells. Based on them, stem cells are first generated, and from them - heart cells,"
says Dr. Carmine Gentile.
Living stem cells are printed on a base that will be "glued" directly onto the areas of the patient's heart damaged during an attack.
Heart Research Institute's cells contract together - "beat" like a real heart.
Pictured: heart cells grown from a tissue sample from a guinea pig.
“They act like a real heart. We were able to make this amazing discovery in our laboratory,"
said Dr. Gentile.
The success of the project could radically change the way doctors treat people with heart attacks. Now patients after a heart attack are treated with angioplasty - to expand blocked or narrowed coronary arteries, a metal mesh balloon is inserted into them, which prevents the artery from sticking together and allows blood to circulate. Doctors also use reperfusion therapy - they prescribe drugs that destroy clots that block arteries. But this treatment is not suitable for all patients, says cardiologist Gemma Figtree of the Colling Institute.
3D printer of the Heart Research Institute.
“We don't know how to replace a healed muscle or what to use for heart regeneration.