3D chemical printer
The 3D printer for chemistry
A new molecule-making machine could do for chemistry what 3D printing did for engineering. It could make it fast, flexible and accessible to anyone.
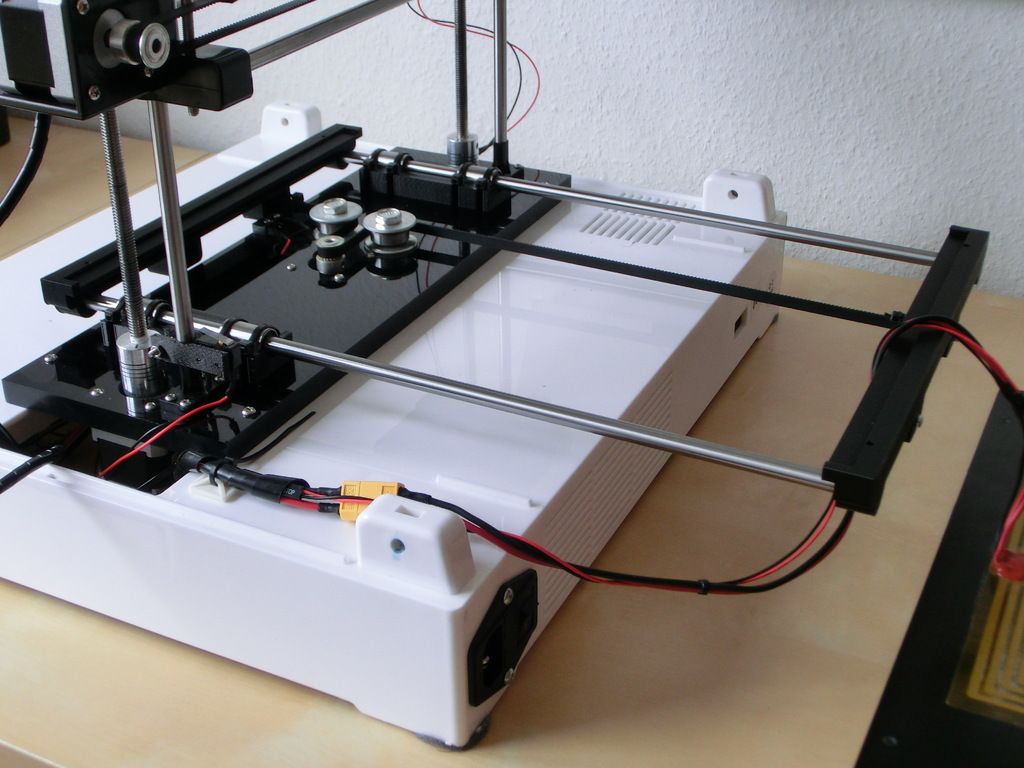
Chemists at the University of Illinois, led by chemistry professor and medical doctor Martin D. Burke, built the machine to assemble complex small molecules at the click of a mouse. It would basically be like a 3D printer at the molecular level. In addition, the automated process has the potential to greatly speed up and enable new drug development. Other technologies that rely on small molecules can also improve.
“We wanted to take a very complex process, chemical synthesis, and make it simple,” said Burke, a Howard Hughes Medical Institute Early Career Scientist. “Simplicity enables automation, which, in turn, can broadly enable discovery and bring the substantial power of making molecules to nonspecialists.”
The researchers described the technology in a paper featured on the cover of the March 13 issue of Science.
What are small molecules
“Small molecules” are a specific class of complex, compact chemical structures found throughout nature. For that reason, they are very important in medicine and in biology as it uncovers the inner workings of cells and tissues. Most medications available now are small molecules. Small molecules also are key elements in technologies like solar cells and LEDs.
However, small molecules are notoriously difficult to make in a lab. Traditionally, an experienced chemist spends years figuring out how to make each one before its function can even be explored. Such a slowdown hinders the development of small-molecule-based medications and technologies.
“Up to now, the bottleneck has been synthesis,” Burke said. “There are many areas where progress is being slowed, and many molecules that pharmaceutical companies aren’t even working on, because the barrier to synthesis is so high.”
The main question that Burke’s group seeks to answer: How do you take something very complex and make it as simple as possible?
The strategy
The group’s strategy has been to break down the complex molecules into smaller building blocks that can be easily assembled. The chemical building blocks all have the same connector piece and can be stitched together with one simple reaction. The process is similar with a child interconnecting plastic blocks that have different shapes but snap all together. Many of the building blocks Burke’s lab has developed are available commercially.
The chemical building blocks all have the same connector piece and can be stitched together with one simple reaction. The process is similar with a child interconnecting plastic blocks that have different shapes but snap all together. Many of the building blocks Burke’s lab has developed are available commercially.
University of Illinois chemistry professor Martin Burke explains what a ”3D printer for chemicals” does. He goes on and says that it can assemble complex small molecules from chemical building blocks. This could aid in rapid drug development.
To automate the building-block assembly, Burke’s group devised a simple catch-and-release method. It adds one building block at a time, rinsing the excess away before adding the next one. They demonstrated that their machine can build 14 different classes of small molecules, including ones with difficult-to-manufacture ring structures. Also, they are using the same automated building-block assembly.
“Dr.
Burke’s research has yielded a significant advance that helps make complex small molecule synthesis more efficient, flexible and accessible,” said Miles Fabian of the National Institutes of Health’s National Institute of General Medical Sciences which partially funded the research. “It is exciting to think about the impact that continued advances in these directions will have on synthetic chemistry and life science research.”
Success is on its way
The automated synthesis technology has been licensed to REVOLUTION Medicines, Inc., a company that Burke co-founded that focuses on creating new medicines based on small molecules found in nature. In addition, the company initially is focusing on anti-fungal medications, an area where Burke’s research has already made strides.
“It is expected that the technology will similarly create new opportunities in other therapeutic areas as well, as the industrialization of the technology will help refine and broaden its scope and scalability,” Burke said.
Perhaps most exciting, this work has opened up an actionable road map to a general and automated way to make most small molecules. Therefore, if that goal can be realized, it will help shift the bottleneck from synthesis to function and bring the power of making small molecules to nonspecialists.
Source: Illinois University
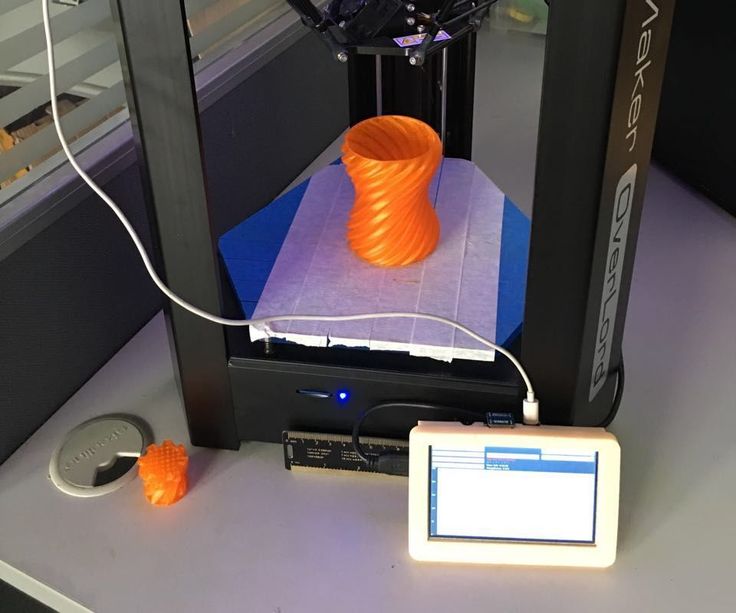
Leapfrog Xeed
Interested to start 3D printing yourself?
Check our professional Leapfrog Xeed 3D printer
This Chemistry 3D Printer Can Synthesize Molecules From Scratch
Say you're a medical researcher interested in a rare chemical produced in the roots of a little-known Peruvian flower. It's called ratanhine, and it's valuable because it has some fascinating anti-fungal properties that might make for great medicines. Getting your hands on the rare plant is hard, and no chemical supplier is or has ever sold it. But maybe, thanks to the work of University of Illinois chemist Martin Burke, you could print it right in the lab.
In a new study published in the journal Science today, Burke has announced the specs of a chemistry's own version of the 3D printer—a machine that can systematically synthesize thousands of different molecules (including the ratanhine molecular family) from a handful of starting chemicals. Such a machine could not only make ratanhine step-by-step, but also could custom-create a dozen other closely-related chemicals—some never even synthesized before by humans. That could allow scientists to test the medicinal properties of a whole molecular family.
"Giving people the ability to synthesize molecules would be game-changing in ways I can't imagine"
"There are many molecules in nature with some extraordinary natural properties, that are incredibly hard to make and just aren't available to be purchased in a [lab supply] catalog," Burke says. "The general assumption has long been that you need a custom strategy to build each molecule, especially if you're trying to automate the process. But we've demonstrated you can use the same system to create radically different molecules. You just need to modulate a step-by-step process."
But we've demonstrated you can use the same system to create radically different molecules. You just need to modulate a step-by-step process."
Media Platforms Design Team
Burke's machine simplifies the complex process of synthesizing chemical into a series of generalizable steps. Whether you're trying to form a ring of carbon atoms or strip away hydrogen atoms, each step requires a dose of starting chemicals, which Burke separates into distinct building blocks. Think of them as simple groups of chemical compounds like O2 or CO2 that snap together.
To perform each step, the machine connects a building block and then induces a chemical reaction and washes away the reaction's byproducts—slowly building each molecule from the ground up. The building blocks are snapped together like LEGOs, allowing the chemicals to mix and a reaction to take place.
Using this process, Burke showed that his machine could manufacture thousands of different chemicals in 14 distinct classes of small molecules, including known medicines to several molecules used in LEDs and solar cells. The amount of time each molecule's synthesis requires is a matter of hours, depending on how many steps are involved.
The amount of time each molecule's synthesis requires is a matter of hours, depending on how many steps are involved.
To answer the question of why such a cool technology is only now becoming available, Burke says the hard part was figuring out the new cleanup method that happens after each chemical reaction. (Some of the information is proprietary, but Burke says he and his colleagues found a universal way to isolate out the molecules they want to keep when washing away the byproducts.)
Media Platforms Design Team
Burke's prototype is currently limited in the number of chemicals it can produce. But he believes that it can already be put to use in developing new drugs. According to Burke, saying his machine can rapidly synthesize some molecules that otherwise would take a trained chemist years to craft.
"And down the line, like the 3D printer, I expect you could see this process in the hands of non-specialists and even consumers," Burke says. "Giving the general population the ability to synthesize these molecules would be [game-changing] in ways I can't even imagine."
"Giving the general population the ability to synthesize these molecules would be [game-changing] in ways I can't even imagine."
William Herkewitz
Science & Technology Reporter
William Herkewitz is a science and technology journalist based in Berlin, Germany. He writes about theoretical physics, AI, astronomy, board games, brewing and everything in between.
3D printing in chemistry - Ananikov Laboratory AnanikovLab.ru
The cheapest, simplest, and most common 3D printing method is Fused Deposition Modeling (FDM). In the vast majority of cases, this method uses the direction of the polymer: the polymer in the form of a thread with a diameter of about 2 mm, wound on a spool, is fed into a small extruder, where it melts and is extruded (pressed) through the nozzle already in the form of a thin thread, from which the layers of the future part are formed. . The nozzle opening diameter and layer thickness together determine the resolution of an FDM printer. The design of such a 3D printer is relatively simple, so these devices are produced by many companies and their cost is low compared to 3D printers designed for printing by other additive methods. A significant advantage of FDM technology is the low cost of consumables, which are various polymers: acrylonitrile butadiene styrene (ABS), biodegradable polylactide (PLA), polyethylene terephthalate (PET), polypropylene (PP), nylon, polycarbonate, etc. nine0003
The design of such a 3D printer is relatively simple, so these devices are produced by many companies and their cost is low compared to 3D printers designed for printing by other additive methods. A significant advantage of FDM technology is the low cost of consumables, which are various polymers: acrylonitrile butadiene styrene (ABS), biodegradable polylactide (PLA), polyethylene terephthalate (PET), polypropylene (PP), nylon, polycarbonate, etc. nine0003
The process of 3D printing of chemical equipment elements from chemically resistant plastic using an FDM 3D printer.
The polymer filament deposition method has an average spatial resolution (usually 0.1 - 0.2 mm) and makes it possible to manufacture parts of various shapes and levels of complexity (Fig. 1). The enormous possibilities of 3D printing make it a very promising technology in education and science. Piece-by-piece production of unique laboratory equipment, visual aids (Fig. 2), mock-ups, models (including working mechanisms with moving parts) - all this is feasible even with the widely available FDM printing method. nine0003
nine0003
Fig. 1. Helical bonded channel made from two different resins in a single 3D print run with two extruders running in sync. Finished products and a 3D model are shown.
Fig. 2. Ball-and-stick model of a molecule made by FDM printing from PLA plastic. To obtain a higher quality, the model was made in parts with subsequent assembly.
The high chemical resistance of some polymers (such as polypropylene, nylon, polyethylene terephthalate) in combination with the FDM printing method is well suited for the manufacture of small chemical laboratory equipment and chemical-technological laboratory installations. However, the real potential of 3D printing is not realized in the manufacture of standard laboratory equipment that can be bought, but in the creation of special products, such as chemical reactors, mixers and other elements of chemical installations, developed in-house for unique experiments. nine0003
The layering technology is mature enough to reproduce even the fine details of small chemical equipment. For example, Figure 3 shows a photograph of a mixer with three inlets and one outlet. The diameter of the internal channels of this mixer is only 2 mm. The conical notches on the inlet pipes are clearly visible, and the height of these notches is 0.5 mm. To increase the mixing efficiency, a miniature helical fin is made inside the central outlet channel. Of course, this is not yet the same thing as shoeing a flea, but everything is moving towards this. The manufacturing time of the mixer was 30 minutes. Try to make such a micro-mixer in half an hour using conventional methods! nine0003
For example, Figure 3 shows a photograph of a mixer with three inlets and one outlet. The diameter of the internal channels of this mixer is only 2 mm. The conical notches on the inlet pipes are clearly visible, and the height of these notches is 0.5 mm. To increase the mixing efficiency, a miniature helical fin is made inside the central outlet channel. Of course, this is not yet the same thing as shoeing a flea, but everything is moving towards this. The manufacturing time of the mixer was 30 minutes. Try to make such a micro-mixer in half an hour using conventional methods! nine0003
Fig. 3. Mixer-tee in the working chamber of a 3D printer, made of polyethylene terephthalate using the FDM method.
Fig. 4. Chemical microreactor with a complex labyrinth channel inside, made of PET by FDM printing. The individual parts of the reactor and the assembly are shown.
The ability to manufacture products with a complex internal structure, as already noted, is one of the main advantages of 3D printing. This possibility is well illustrated in Figure 4, which shows a complete chemical reactor the size of a matchbox. Like the "adult" chemical reactor, this "baby" has a shell, a lid connected by bolts to the shell, and a gasket to seal the working space. It is enough to look under the lid to see the complexity of the inner world of this worker of science: a labyrinth channel is organized inside the reactor, forcing the reaction mixture entering the shell through the side pipe to move along a complex trajectory, so that the reaction time is optimal to obtain the desired products. There are small baffles at the bottom of the labyrinth channel to keep the finely dispersed catalyst from being washed out quickly. The reaction products leave the reactor through the lower branch pipe. nine0003
This possibility is well illustrated in Figure 4, which shows a complete chemical reactor the size of a matchbox. Like the "adult" chemical reactor, this "baby" has a shell, a lid connected by bolts to the shell, and a gasket to seal the working space. It is enough to look under the lid to see the complexity of the inner world of this worker of science: a labyrinth channel is organized inside the reactor, forcing the reaction mixture entering the shell through the side pipe to move along a complex trajectory, so that the reaction time is optimal to obtain the desired products. There are small baffles at the bottom of the labyrinth channel to keep the finely dispersed catalyst from being washed out quickly. The reaction products leave the reactor through the lower branch pipe. nine0003
Microreactors can consist of any number of elements, each of which is individually manufactured using 3D printing (Fig. 5). Individual elements (Fig. 6) are eventually combined into workable chemical plants (Fig. 7).
7).
Fig. 5. Microreactor, consisting of a shell, a lid, a catalytic cartridge and a replaceable nozzle. The individual parts of the reactor, the reactor assembly and a three-dimensional model of the assembly are shown.
nine0003
Fig. 6. Mixer and zigzag microreactor made of PET.
Fig. 7. Laboratory chemical plant assembled from a microreactor and mixer, created by FDM printing methods.
3D printing can significantly speed up experimental chemical research, because it makes it possible to manufacture even complex multi-component chemical equipment right in the laboratory without significant material costs. This applies to both fundamental chemical research and chemical engineering projects. For chemical technology, 3D printing provides a truly unique opportunity to produce a series of reactors or other equipment with different design parameters in a short time to find the optimal solution. The cost of manufacturing even entire series of products using FDM printing is insignificant compared to the cost of commercial laboratory equipment. Even now, this technology can act as a full-fledged tool in the creation of scientific chemical equipment. nine0003
Even now, this technology can act as a full-fledged tool in the creation of scientific chemical equipment. nine0003
An example of the use of 3D printing in the creation of a photochemical reactor:
"Visible Light Mediated Metal-free Thiol–yne Click Reaction", Chem. sci. , 2016 , 7, 6740-6745, DOI: 10.1039/C6SC02132H. Online Link: http://dx.doi.org/10.1039/c6Sc02132H
>
3D-rendering in chemistry
Application
Subscribe to
Sign
I do not want
12
Good morning. .. Monday)) :D
I found such an interesting case on the use of 3D printing on the Internet, I decided to post it, since it has not been here yet.
'FDM has a medium spatial resolution (typically 0.1 - 0.2 mm) and makes it possible to manufacture parts of various shapes and levels of complexity. The enormous possibilities of 3D printing make it a very promising technology in education and science. Individual production of unique laboratory equipment, visual aids, layouts, models (including working mechanisms with moving parts) - all this is feasible even with the widely available FDM printing method. nine0003
Individual production of unique laboratory equipment, visual aids, layouts, models (including working mechanisms with moving parts) - all this is feasible even with the widely available FDM printing method. nine0003
Helical bonded channel made from two different resins in a single 3D print run with two extruders running in sync. Finished products and a 3D model are shown.
Ball-and-stick model of a molecule made by FDM printing from PLA plastic. To obtain a higher quality, the model was made in parts with subsequent assembly.
The high chemical resistance of some polymers (such as polypropylene, nylon, polyethylene terephthalate) in combination with the FDM printing method is well suited for the manufacture of small chemical laboratory equipment and chemical-technological laboratory installations. However, the real potential of 3D printing is not realized in the manufacture of standard laboratory equipment that can be bought, but in the creation of special products, such as chemical reactors, mixers and other elements of chemical installations, developed in-house for unique experiments. nine0003
nine0003
Layering technology -- mature enough to reproduce even the fine details of small chemical equipment. For example, the figure shows a photograph of a mixer with three inlets and one outlet. The diameter of the internal channels of this mixer is only 2 mm. The conical notches on the inlet pipes are clearly visible, and the height of these notches is 0.5 mm. To increase the mixing efficiency, a miniature helical fin is made inside the central outlet channel. nine0003
Mixer-tee in the working chamber of a 3D printer, made of polyethylene terephthalate
Chemical microreactor with a complex labyrinth channel inside, made of PET by FDM printing.
The ability to manufacture products with a complex internal structure, as already noted, is one of the main advantages of 3D printing. This possibility is well illustrated in the photo above, which shows a complete chemical reactor the size of a matchbox. Like the "adult" chemical reactor, this "baby" has a shell, a lid connected by bolts to the shell, and a gasket to seal the working space. It is enough to look under the lid to see the complexity of the inner world of this worker of science: a labyrinth channel is organized inside the reactor, forcing the reaction mixture entering the shell through the side pipe to move along a complex trajectory, so that the reaction time is optimal to obtain the desired products. There are small baffles at the bottom of the labyrinth channel to keep the finely dispersed catalyst from being washed out quickly. The reaction products leave the reactor through the lower branch pipe. nine0003
It is enough to look under the lid to see the complexity of the inner world of this worker of science: a labyrinth channel is organized inside the reactor, forcing the reaction mixture entering the shell through the side pipe to move along a complex trajectory, so that the reaction time is optimal to obtain the desired products. There are small baffles at the bottom of the labyrinth channel to keep the finely dispersed catalyst from being washed out quickly. The reaction products leave the reactor through the lower branch pipe. nine0003
Microreactors can be made up of any number of elements, each individually 3D printed.
Microreactor, consisting of a shell, a cover, a catalytic cartridge and a replaceable nozzle
A mixer and a zigzag microreactor made of PET
A laboratory chemical plant assembled from a microreactor and a mixer
3D printing can significantly speed up experimental chemical research, because makes it possible to manufacture even complex multicomponent chemical equipment directly in the laboratory without significant material costs.