What is 3d organ printing
3D-printed organs: The future of transplantation
CNN —
What if doctors could just print a kidney, using cells from the patient, instead of having to find a donor match and hope the patient’s body doesn’t reject the transplanted kidney?
The soonest that could happen is in a decade, thanks to 3D organ bioprinting, said Jennifer Lewis, a professor at Harvard University’s Wyss Institute for Biologically Inspired Engineering. Organ bioprinting is the use of 3D-printing technologies to assemble multiple cell types, growth factors and biomaterials in a layer-by-layer fashion to produce bioartificial organs that ideally imitate their natural counterparts, according to a 2019 study.
This type of regenerative medicine is in the development stage, and the driving force behind this innovation is “real human need,” Lewis said.
In the United States, there are 106,075 men, women and children on the national organ transplant waiting list as of June 10, according to the Health Resources & Services Administration. However, living donors provide only around 6,000 organs per year on average, and there are about 8,000 deceased donors annually who each provide 3.5 organs on average.
47-year-old Steve Verze is to become the first man in the world to be fitted with a 3D printed eye, according to Moorfields Eye Hospital.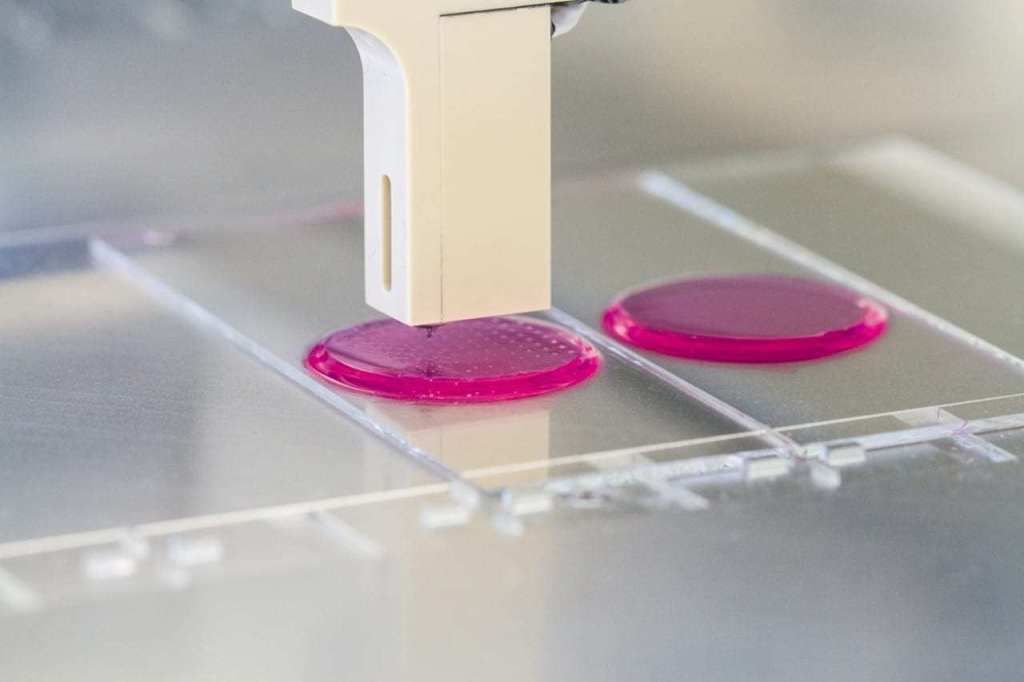 He tried the eye for size earlier this month, as photographed here.
He tried the eye for size earlier this month, as photographed here.
British man given 3D printed eye in world first, hospital says
The cause of this discrepancy is “a combination of people who undergo catastrophic health events, but their organs aren’t high enough quality to donate, or they’re not on the organ donor list to begin with, and the fact that it’s actually very difficult to find a good match” so the patient’s body doesn’t reject the transplanted organ, Lewis said.
And even though living donors are an option, “to do surgery on someone who doesn’t need it” is a big risk, said Dr.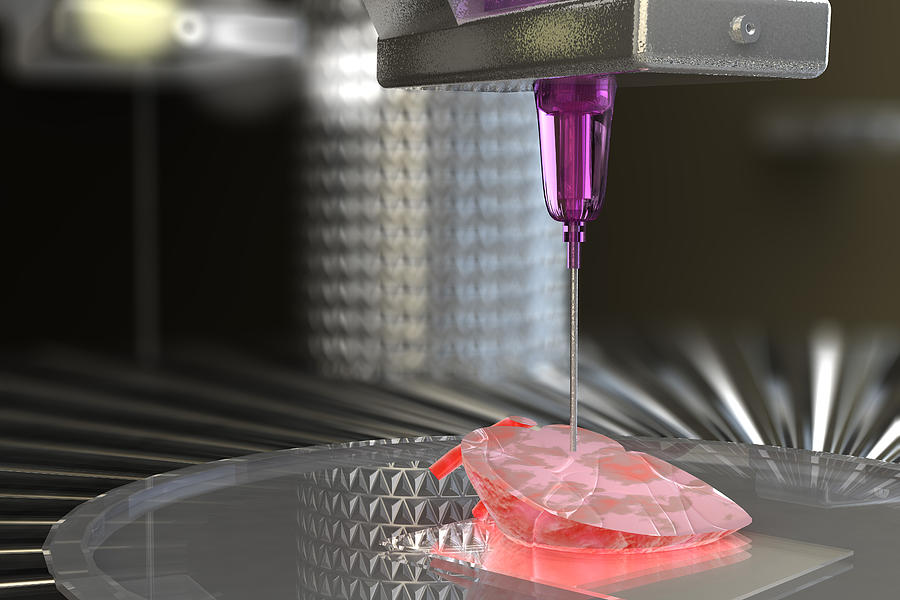 Anthony Atala, director of the Wake Forest Institute for Regenerative Medicine. “So, living related donors are usually not the preferred way to go because then you’re taking an organ away from somebody else who may need it, especially now as we age longer.”
Anthony Atala, director of the Wake Forest Institute for Regenerative Medicine. “So, living related donors are usually not the preferred way to go because then you’re taking an organ away from somebody else who may need it, especially now as we age longer.”
Atala and his colleagues were responsible for growing human bladders in a lab by hand in 2006, and implanting a complicated internal organ into people for the first time – saving the lives of three children in whom they implanted the bladders.
A bladder scaffold is seeded with cells at the Wake Forest Institute for Regenerative Medicine.
Courtesy Wake Forest Institute for Regenerative Medicine Every day, 17 people die waiting for an organ transplant, according to the Health Resources & Services Administration. And every nine minutes, another person is added to the waitlist, the agency says. More than 90% of the people on the transplant list in 2021 needed a kidney.
And every nine minutes, another person is added to the waitlist, the agency says. More than 90% of the people on the transplant list in 2021 needed a kidney.
“About a million people worldwide are in need of a kidney. So they have end-stage renal failure, and they have to go on dialysis,” Lewis said. “Once you go on dialysis, you have essentially five years to live, and every year, your mortality rate increases by 15%. Dialysis is very hard on your body. So this is really motivating to take on this grand challenge of printing organs.”
“Anti-hypertensive pills are not scarce. Everybody who needs them can get them,” Martine Rothblatt, CEO and chairman of United Therapeutics, said at the Life Itself conference, a health and wellness event presented in partnership with CNN.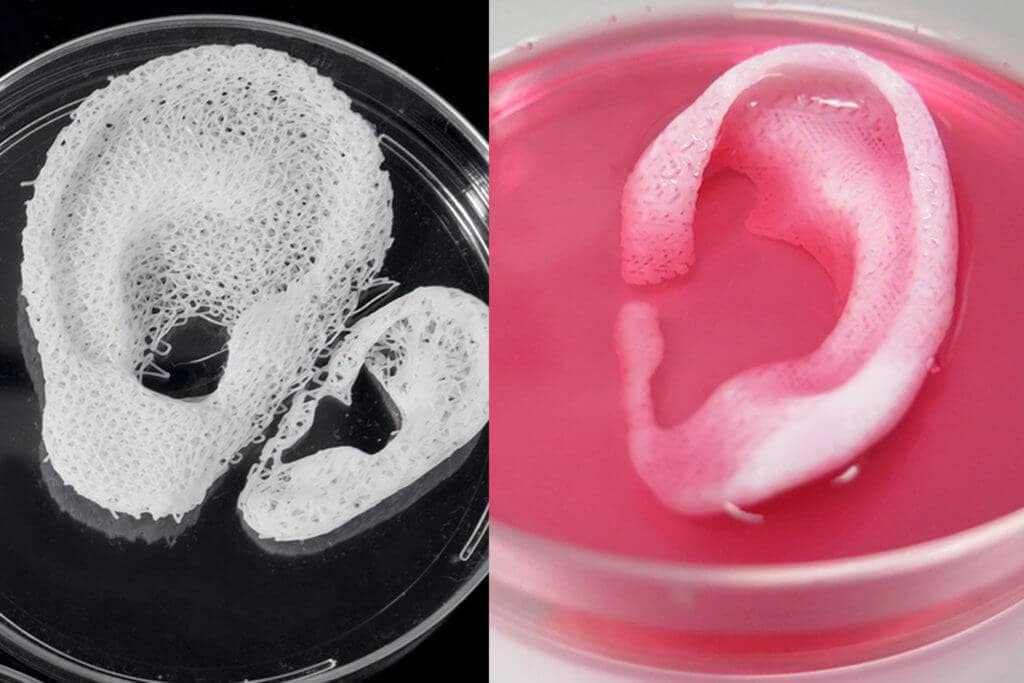 United Therapeutics is one of the conference’s sponsors.
United Therapeutics is one of the conference’s sponsors.
“There is no practical reason why anybody who needs a kidney – or a lung, a heart, a liver – should not be able to get one,” she added. “We’re using technology to solve this problem.”
To begin the process of bioprinting an organ, doctors typically start with a patient’s own cells. They take a small needle biopsy of an organ or do a minimally invasive surgical procedure that removes a small piece of tissue, “less than half the size of a postage stamp,” Atala said. “By taking this small piece of tissue, we are able to tease cells apart (and) we grow and expand the cells outside the body. ”
”
This growth happens inside a sterile incubator or bioreactor, a pressurized stainless steel vessel that helps the cells stay fed with nutrients – called “media” – the doctors feed them every 24 hours, since cells have their own metabolism, Lewis said. Each cell type has a different media, and the incubator or bioreactor acts as an oven-like device mimicking the internal temperature and oxygenation of the human body, Atala said.
“Then we mix it with this gel, which is like a glue,” Atala said. “Every organ in your body has the cells and the glue that holds it together. Basically, that’s also called ‘extracellular matrix. ’ ”
’ ”
Richard Roth with Samira Jafari
Courtesy Samira JafariLiving organ donations save lives. This is how you become a donor
This glue is Atala’s nickname for bioink, a printable mixture of living cells, water-rich molecules called hydrogels, and the media and growth factors that help the cells continue to proliferate and differentiate, Lewis said. The hydrogels mimic the human body’s extracellular matrix, which contains substances including proteins, collagen and hyaluronic acid.
The non-cell sample portion of the glue can be made in a lab, and “is going to have the same properties of the tissue you’re trying to replace,” Atala said.
The biomaterials used typically have to be nontoxic, biodegradable and biocompatible to avoid a negative immune response, Lewis said. Collagen and gelatin are two of the most common biomaterials used for bioprinting tissues or organs.
From there, doctors load each bioink – depending on how many cell types they’re wanting to print – into a printing chamber, “using a printhead and nozzle to extrude an ink and build the material up layer by layer,” Lewis said. Creating tissue with personalized properties is enabled by printers being programmed with a patient’s imaging data from X-rays or scans, Atala said.
“With a color printer you have several different cartridges, and each cartridge is printing a different color, and you come up with your (final) color,” Atala added. Bioprinting is the same; you’re just using cells instead of traditional inks.
How long the printing process takes depends on several factors, including the organ or tissue being printed, the fineness of the resolution and the number of printheads needed, Lewis said. But it typically lasts a few to several hours. The time from the biopsy to the implantation is about four to six weeks, Atala said.
A 3D printer seeds different types of cells onto a kidney scaffold at the Wake Forest Institute for Regenerative Medicine.
The ultimate challenge is “getting the organs to actually function as they should,” so accomplishing that “is the holy grail,” Lewis said.
“Just like if you were to harvest an organ from a donor, you have to immediately get that organ into a bioreactor and start perfusing it or the cells die,” she added. To perfuse an organ is to supply it with fluid, usually blood or a blood substitute, by circulating it through blood vessels or other channels.
Depending on the organ’s complexity, there is sometimes a need to mature the tissue further in a bioreactor or further drive connections, Lewis said. “There’s just a number of plumbing issues and challenges that have to be done in order to make that printed organ actually function like a human organ would in vivo (meaning in the body). And honestly, this has not been fully solved yet.”
“There’s just a number of plumbing issues and challenges that have to be done in order to make that printed organ actually function like a human organ would in vivo (meaning in the body). And honestly, this has not been fully solved yet.”
Once a bioprinted organ is implanted into a patient, it will naturally degrade over time – which is OK since that’s how it’s designed to work.
“You’re probably wondering, ‘Well, then what happens to the tissue? Will it fall apart?’ Actually, no,” Atala said. “These glues dissolve, and the cells sense that the bridge is giving way; they sense that they don’t have a firm footing anymore. So cells do what they do in your very own body, which is to create their own bridge and create their own glue.”
So cells do what they do in your very own body, which is to create their own bridge and create their own glue.”
Atala and Lewis are conservative in their estimates about the number of years remaining before fully functioning bioprinted organs can be implanted into humans.
“The field’s moving fast, but I mean, I think we’re talking about a decade plus, even with all of the tremendous progress that’s been made,” Lewis said.
“I learned so many years ago never to predict because you’ll always be wrong,” Atala said. “There’s so many factors in terms of manufacturing and the (US Food and Drug Administration regulation). At the end of the day, our interest, of course, is to make sure the technologies are safe for the patient above all.”
“There’s so many factors in terms of manufacturing and the (US Food and Drug Administration regulation). At the end of the day, our interest, of course, is to make sure the technologies are safe for the patient above all.”
Whenever bioprinting organs becomes an available option, affordability for patients and their caregivers shouldn’t be an issue.
They’ll be “accessible for sure,” Atala said. “The costs associated with organ failures are very high. Just to keep a patient on dialysis is over a quarter of a million dollars per year, just to keep one patient on dialysis. So, it’s a lot cheaper to create an organ that you can implant into the patient. ”
”
The average kidney transplant cost was $442,500 in 2020, according to research published by the American Society of Nephrology – while 3D printers retail for around a few thousand dollars to upward of $100,000, depending on their complexity. But even though low-cost printers are available, pricey parts of bioprinting can include maintaining cell banks for patients, culturing cells and safely handling biological materials, Lewis said.
Some of the major costs of current organ transplantation are “harvesting the organ from the donor, the transport costs and then, of course, the surgery that the recipient goes through, and then all the care and monitoring,” Lewis said. “Some of that cost would still be in play, even if it was bioprinted.”
“Some of that cost would still be in play, even if it was bioprinted.”
Printing the future: 3D bioprinters and their uses
First things first … understanding 3D printers
Traditional printers—like the type you have in your home or office—work in two dimensions. That is, they are able to print text or images on a flat surface (usually paper), using the x (horizontal) and y (vertical) dimensions. 3D printers add another dimension—depth (z). They can move up and down, left and right, and backwards and forwards and, instead of delivering ink on paper, they distribute different materials—ranging from polymers (including plastics), metal, ceramics, even chocolate—to ‘print’ an item layer by layer in a process that is known as ‘ additive manufacturing GLOSSARY additive manufacturingA process by which digital 3D design data is used to build up a component in layers by depositing material. The term ‘3D printing’ is increasingly used as a synonym for additive manuafcturing. ’.
’.
To create a 3D object, you first need a blueprint—that is, a digital file created using modelling software. Once created, the computer-generated model is sent to the printer. Your chosen material (such as plastic) is loaded into the device, ready to be heated to allow it to easily flow from the printer nozzle. As it reads the blueprint, the printer head moves up and down, side to side and forward and back, depositing successive layers of the chosen material to build up your final product. As each layer is printed it transforms into a solid form, either by cooling, chemical reaction (often induced by light), or by the mixing of two different solutions delivered by the printer head. New layers adhere to the previous one to create a stable, cohesive item. Almost any shape can be created in this way, including moving parts and complex layers.
A wide range of items are already being created using 3D printers, including jewellery, clothing, toys, prototypes, camera cases, and high-end manufactured items.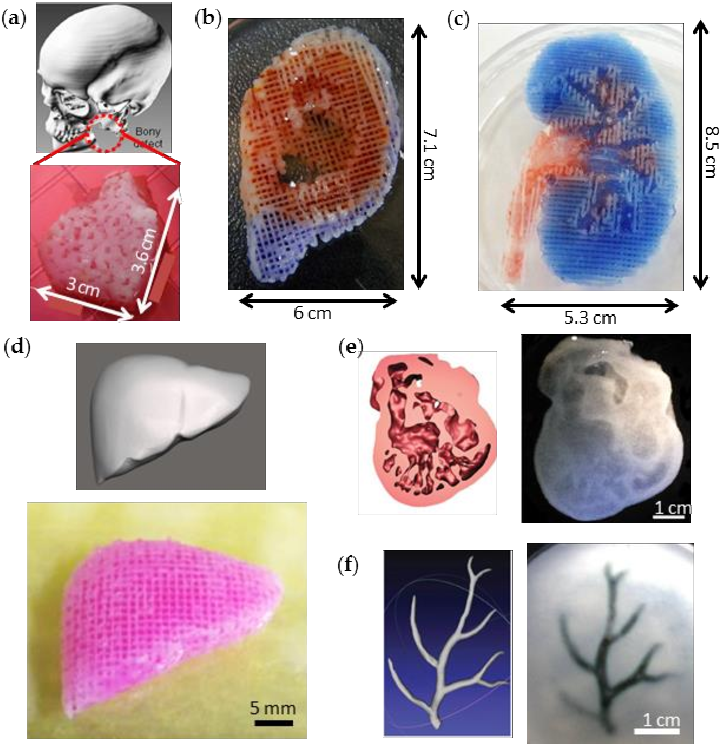
3D printing allows us to produce objects with geometric structures that would be difficult or impossible to create by other means. Image source: TED Conference / Flickr.
Bioprinting
Bioprinters work in almost the exact same way as 3D printers, with one key difference. Instead of delivering materials such as plastic, ceramic, metal or food, they deposit layers of biomaterial, that may include living cells, to build complex structures like blood vessels or skin tissue.
Hang on …living cells? Where do they get those? Well, every tissue in the body is naturally made up of different cell types. So the required cells (kidney cells, skin cells and so on) are taken from a patient and then cultivated until there are enough to create the ‘bio-ink’, which is loaded into the printer. This is not always possible, so, for some tissues, adult stem cells—which can develop to form the cells required in different tissues—can be used.
Following detailed computer designs and models, often based on scans taken directly from a patient, precision printer heads deposit cells exactly where they are needed and, over the course of several hours, an organic object is built up using a large number of very thin layers.
Of course, you generally need more than just cells, so most bioprinters also deliver some sort of organic or synthetic ‘glue’—a dissolvable gel, collagen scaffold or other type of support that the cells can attach to and grow on. This helps them to mould and stabilise into the correct form. Amazingly, some cells can assume the correct positioning by themselves with no scaffolding.
How do they know where to go? They use their inherent properties to seek out similar cells to join with. They innately know where they are needed, like the way cells in an embryo develop in the womb, or tissue in an adult moves to repair damage. Researchers are then able to control the shape in which they do that, with the printer building the final structure.
There are many different printers on the market and undergoing testing, and therefore methods for delivering these cells and biomaterials also differ. Some of the more commonly used methods at present include extrusion GLOSSARY extrusionTechnique where continuous filaments of a material are forced through a nozzle in a controlled manner to construct a 3D structure
, laser GLOSSARY laserIn laser-guided direct writing method, photons from a laser beam trap and guide cells by exploiting the differences in refractive indexes of cells and cell media.
, microvalves GLOSSARY microvalvesThe bioink droplet is generated by the opening and closing of a microvalve under constant pneumatic pressure.
, inkjet GLOSSARY inkjetDrop-based bioprinting (inkjet) creates cellular constructs using individual droplets of a designated material, which may also be combined with a cell line. and tissue fragment GLOSSARY tissue fragmentThe combination of bioprinting techniques with biological self-assembly. This approach exploits the intrinsic capacity of closely spaced tissue fragments to fuse together, otherwise known as tissue fluidity.
printing.
and tissue fragment GLOSSARY tissue fragmentThe combination of bioprinting techniques with biological self-assembly. This approach exploits the intrinsic capacity of closely spaced tissue fragments to fuse together, otherwise known as tissue fluidity.
printing.
Each method has its own advantages and drawbacks, and each presents unique challenges to be overcome. Researchers predict it will be the combination of several of these techniques that will provide the most significant advancements in bioprinting. A more detailed explanation of these methods is available from the University of Wollongong.
So, essentially, scientists and medical researchers are using scanners and printers traditionally reserved for auto design, model building and product prototyping to instead create living human tissues.
Universities, researchers and private companies around the world are all involved in advancing bioprinting technologies. Let’s take a look at some of the things they’re working on.
Let’s take a look at some of the things they’re working on.
Bone
Every year, more than 2.2 million people worldwide require bone-graft procedures to repair bone defects.
Current bone grafts often use a synthetic cement-based material combined with a patient’s own bone. Limitations with these materials have resulted in structures that do not have appropriate mechanical integrity or allow for the creation of new tissue. Replicating the naturally occurring bone–cartilage interface has also been problematic.
However, a team from Swansea University has developed a bioprinting process that can create an artificial bone matrix in the exact shape of the bone required, using a biocompatible material that is both durable and regenerative. These newly printed ‘bones’ are transplanted into the body where, over a period of several months, they fuse with and are eventually replaced by a patient’s natural bones with few, if any, complications.
To print a small bone with trabecular GLOSSARY trabecularCancellous bone, also known as spongy or trabecular bone, is one of the two types of bone tissue found in the human body. Cancellous bone is found at the ends of long bones, as well as in the pelvic bones, ribs, skull and the vertebrae in the spinal column.
features currently takes around two hours. With these short time frames, surgeons could soon be printing them in the operating theatre as they work.
Cancellous bone is found at the ends of long bones, as well as in the pelvic bones, ribs, skull and the vertebrae in the spinal column.
features currently takes around two hours. With these short time frames, surgeons could soon be printing them in the operating theatre as they work.
Other researchers at the University of Nottingham in England are working on similar bone replacements. They bioprint an exact copy of the part of the bone they are trying to replace—this becomes the scaffold. This is then coated with adult human stem cells—capable of developing into almost any different tissue type. This is combined with bio-ink from the printer—a combination of polylactic acid (which provides the mechanical strength of bone) and alginate—a gel-like substance that works as a cushioning material for the cells. The final product is then implanted into the body, where, within about three months, the scaffold will disappear and be replaced by new bone.
Researchers hope that, in the future, bioprinted bones could be created with enough reliability to underpin intricate spinal reconstruction, and that the bone material could be further improved to enhance its compatibility with cartilage cells.
Skin
If a person is badly burned, healthy skin can be taken from another part of the body and used to cover the affected area. Sometimes, however, there is not enough undamaged skin to harvest.
Researchers working at the Wake Forest School of Medicine have successfully designed, built and tested a printer that can print skin cells directly onto a burn wound. A scanner first determines the size and depth of the wound, and this information is passed to the printer, which, after the relevant cells have been cultivated, applies the correct cell types at the correct depth to cover the wound. Unlike traditional skin grafts, you only require a patch of skin one-tenth the size of the burn to be able to grow enough skin cells for skin printing. While this technology is still in the experimental stage, researchers hope it will be widely available within the next five years.
As mentioned, 3D printers print in layers, and because skin is a multilayered organ with different cell types, it’s well suited to this type of technology. One American company has already produced multilayered skin consisting of the dermis and epidermis layers. There are still many challenges ahead though, particularly how to stop the heat generated by the printer from damaging the cells or their viability. And of course, as with most parts of the human body, skin is more complex than it first appears—there are nerves, blood vessels, and a myriad of other aspects that need to be accommodated.
One American company has already produced multilayered skin consisting of the dermis and epidermis layers. There are still many challenges ahead though, particularly how to stop the heat generated by the printer from damaging the cells or their viability. And of course, as with most parts of the human body, skin is more complex than it first appears—there are nerves, blood vessels, and a myriad of other aspects that need to be accommodated.
Blood vessels
Considering there are tens of thousands of kilometres of veins, arteries and capillaries in your body, it’s good news that researchers have been working on ways to replace them should they ever fail. Creating viable blood vessels is also essential to get all those other potential bioprinted body parts to work properly.
Research engineer Monica Moya from Lawrence Livermore National Laboratory is using bioprinting to create ‘living’ blood vessels. The materials and environment created by her bioprinters are engineered in such a way as to enable small blood vessels—human capillaries—to develop on their own. This development takes a while so, to assist, tubes of cells and other biomaterials are printed out to help deliver vital nutrients to the surrounding printed environment. Over a period of time, the self-assembled capillaries connect with the bioprinted tubes, thereby beginning to deliver nutrients to the cells on their own—mimicking the way these structures work in the human body.
This development takes a while so, to assist, tubes of cells and other biomaterials are printed out to help deliver vital nutrients to the surrounding printed environment. Over a period of time, the self-assembled capillaries connect with the bioprinted tubes, thereby beginning to deliver nutrients to the cells on their own—mimicking the way these structures work in the human body.
If you take this approach of co-engineering with nature you allow biology to help create the finer resolution of the printed tissue … We’re leveraging the body’s ability for self-directed growth, and you end up with something that is more true to physiology. We can put the cells in an environment where they know, ‘I need to build blood vessels.’ With this technology we guide and orchestrate the biology.Monica Moya, Research engineer
Having successfully created an ‘unorganised’ network of blood vessels, she and her team are now focusing on creating a tiered hierarchy such as that which exists in the human body.
Another method of creating blood vessels has been developed by researchers at the Brigham and Women’s Hospital. Using an agarose fibre template covered with hydrogel, the team was able to construct microchannel networks which exhibited various architectural features.
Internal organs
There are currently around 1,600 people on the organ transplant waiting list in Australia. Many researchers hope that, within 20 years, these lists will be a thing of the past. They imagine a world in which an organ can be printed and transplanted in just a few hours, without rejection from the body. These printed organs will be created from the very cells of the body they will re-enter, matching the exact size, specifications and requirements of each individual patient.
At present, the bioprinting of fully functional complex internal organs (such as hearts, kidneys and livers) is still at least 10 years away, possibly more, but advancements are occurring at a rapid rate.
Less complex organs—such as bladders—are already possible. At the Wake Forest University in the United States, researchers have successfully taken cells from a patient’s original, poorly functioning bladder, cultivated them and added additional nutrients. A 3D mould of the patient’s bladder was then printed and the cultivated cells soaked through it. The mould was placed in an incubator—a bit like baking a cake—and, when it came out, was able to be transplanted into the patient’s body. The mould will eventually break down, leaving only the organic material behind.
The same team have also successfully produced viable urethras. Once again, a combination of a patient’s own cells and additional nutrients is seeded with scaffolding and then sewn into the correct shape of a urethra. These engineered parts can then transplanted back into the patient’s body.
While bioengineers have successfully printed a tiny (1 millimetre thick x 4 millimetres wide) 3D functioning liver, it survived for only five days. It’s a start, but there’s a long way to go.
It’s a start, but there’s a long way to go.
Kidneys are also incredibly difficult as they contain complex cellular processes for filtration—not something that can be easily replicated. Researchers in Australia have used human stem cells to grow a kidney organoid that contains all the required cell types for a kidney. Such cells may provide a valuable starting source for bioprinting a more complex kidney structure.
Dr Anthony Atala shows a kidney that was printed on stage at TED. Image source: Steve Jurvetson / Flickr.Amazingly, hearts may be one of the easier organs to make, as they’re essentially a pump with tubes. Of course, it’s not quite as simple as that, but many researchers believe that we’ll have transplantable biohearts before kidneys or livers. Researchers in the United States have created ‘organoids’—3D printed, beating cardiac cells, which are shaped using a specialised 3D printer and are, essentially, fully functional.
While it’s easy to print a full replica of an organ, it’s more difficult to make it ‘real’, with blood vessels, connecting tissues, nerves, filtration systems, strength, durability and all the aspects that go in to making our ‘natural’ organs so complex.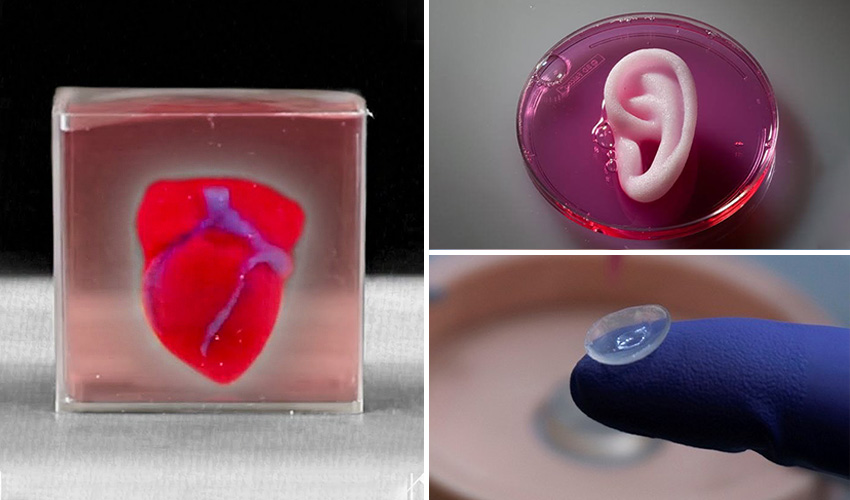 These challenges aren’t putting off the many researchers who are working to find solutions to these intricate problems.
These challenges aren’t putting off the many researchers who are working to find solutions to these intricate problems.
Human cartilage
The 3D printing of human cartilage should, researchers hope, lead to implantable replacements for trauma victims who need reconstructive surgery. Ear sliced off? Nose smashed? No worries—scientists in Zurich have developed a process that could enable hospitals to print a full-sized human nose implant in under 20 minutes. They believe that any cartilage implant could be produced with their new process. In an article, researcher Matti Kesti described the technology:
‘A serious car accident results in a passenger’s nose being shattered. It is possible to reconstruct this as a 3D model on the computer. At the same time, a biopsy is performed on the patient and cartilage cells removed from his or her own body, for example from the knee, finger, ear or splinters of the shattered nose. The cells are spawned in the laboratory and mixed with a biopolymer. From this toothpaste-like suspension, a nose cartilage transplant is created using the bioprinter, which is implanted in the patient during surgery. In this process, the biopolymer is used merely as a form of shaping mould; it is subsequently broken down by the body’s own cartilage cells. After a couple of months, it is impossible to distinguish between the transplant and the body’s own nose cartilage.’
From this toothpaste-like suspension, a nose cartilage transplant is created using the bioprinter, which is implanted in the patient during surgery. In this process, the biopolymer is used merely as a form of shaping mould; it is subsequently broken down by the body’s own cartilage cells. After a couple of months, it is impossible to distinguish between the transplant and the body’s own nose cartilage.’
As the implant was grown from the body’s own cells, the risk of rejection is far lower than for an implant made of, say, silicone. An added benefit is that the cellular implant grows together with the patient, because it is controlled by the patient’s internal growth engine, as is the case for other body parts. This is particularly important for younger implant-recipients.
Video: 3D printing with living “ink” (Cornell University / YouTube). View details and transcript.
A bonus use: drug testing and medical research
One of the key potential areas for using bioprinted living materials is in the arena of medical testing, drug research and safety. Many of these bioprinted tissues share common features with native tissues—such as the presence of multiple cells types, cellular density and key architectural features. In this way, researchers are able to study the effects of different diseases, disease progression and possible treatments in a native microenvironment.
One of the most exciting recent advances is the idea of a ‘benchtop brain’, which has been developed by researchers at the ARC Centre of Excellence for Electromaterials Science (ACES). They have developed a 3D printed, six-layered structure that incorporates neural cells that mimic the structure of brain tissue.
This has huge potential benefits for researchers, pharmaceutical companies and private companies. It will enable them to test new products and drugs on tissue that accurately reflects human brain tissue, as opposed to animal specimens which can produce a very different reaction. The benchtop brain could also be used to further research into brain disorders such as schizophrenia or Alzheimer’s disease.
The benchtop brain could also be used to further research into brain disorders such as schizophrenia or Alzheimer’s disease.
We are still a long way from printing a brain but the ability to arrange cells so as they form neuronal networks is a significant step forwardACES Director and research author Professor Gordon Wallace
By allowing researchers to work with ‘real-time’ human tissue, it could greatly speed up testing processes, and produce more realistic, accurate results. It could also help reduce the need to use lab animals for medical tests, as well as potentially dangerous trials on humans.
Mobile 3D printing
A spin-off from the traditional bioprinter is the BioPen, developed by Australian researchers and led by Professor Gordon Wallace at the University of Wollongong. The pen functions in a similar way to a bioprinter, but is far more mobile. It combines cellular material with protective gels, which are delivered via the pen head as the surgeon ‘draws’ with the ink directly on the damaged segment of bone or cartilage to repair any damage. As each layer is ‘drawn’ by the surgeon, it is exposed to UV light from a source attached to the pen. This hardens the gel so that additional layers can be added, eventually creating a three-dimensional framework.
As each layer is ‘drawn’ by the surgeon, it is exposed to UV light from a source attached to the pen. This hardens the gel so that additional layers can be added, eventually creating a three-dimensional framework.
The pen will give surgeons a greater degree of accuracy and control over where they place biomaterial, reduce time in surgery and accelerate the regeneration of bone and cartilage. Once the biomaterial is delivered to the exact site determined by the surgeon, the protective gels gradually degrade away, replaced by the new tissue which begins to repair the area.
Professor Peter Choong, BioPen co-developer and director of orthopaedics at St Vincent’s Hospital Melbourne, has been using the pen in clinical trials since 2013. He noted, ‘Professor Wallace's research team brings together the science of stem cells and polymer chemistry to help surgeons design and personalise solutions for reconstructing bone and joint defects in real time’.
The BioPen allows surgeons to draw bio-ink directly onto damaged bone or cartilage to repair damage.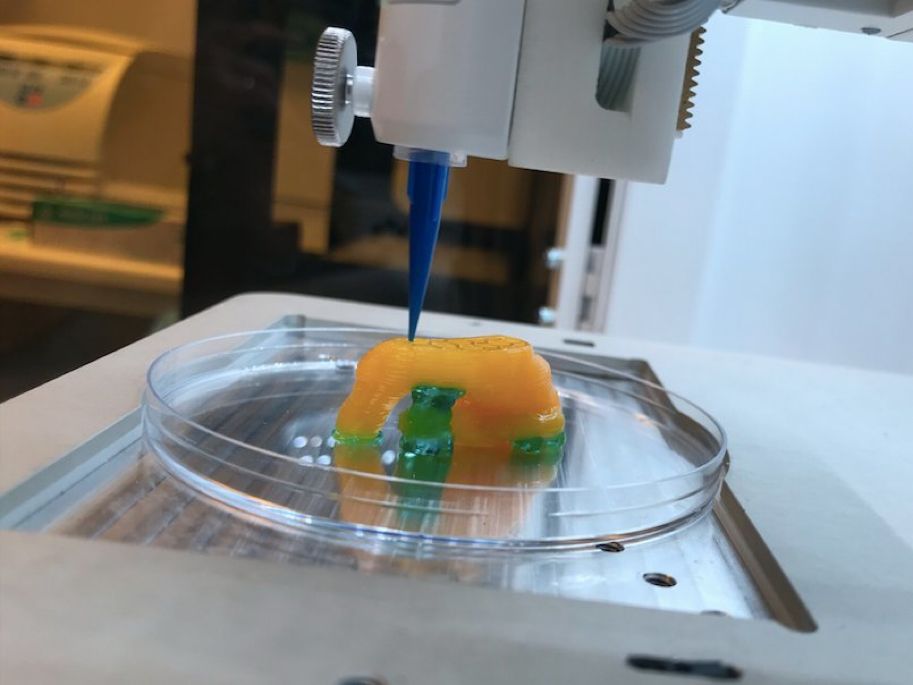 Image source: University of Wollongong; used with permission.
Image source: University of Wollongong; used with permission.Why is it taking so long?
The human body and its various components are a lot more complicated than a plastic toy or a ceramic piece of jewellery. A human organ has a complex web of cells, tissues, nerves and structures that need to be correctly positioned for the organ to function properly. From arranging the thousands of tiny capillaries in a liver, to actually getting a printed heart to ‘beat’ and contract—it’s a long, difficult process. While some parts of the human body are more complex than others—an ear or nose is easier to make than a bladder or a urethra, while the larger organs like kidneys, heart and liver are at the top of the difficulty rating—each piece has its own specialised requirements and issues that need addressing. Added to this is the selection and availability of the right materials, cell types, and creating bio-inks that meet the dual demands of both printability and cell functionality. There is also the need to take in to account different growth rates and differentiation factors, as well as developing the right software and hardware to make it all come together.
There is also the need to take in to account different growth rates and differentiation factors, as well as developing the right software and hardware to make it all come together.
Even after researchers believe they have it all ‘right’, bioprinted technologies—like all new medical treatments—are still required to pass safety tests and proper regulatory processes before they can be made available to the public.
Working through all these complexities requires the integration and input of technologies from fields including engineering, biomaterials science, cell biology, physics and medicine. So, you’ll just have to be a little more patient!
Conclusion
Given its interdisciplinary nature, 3D bioprinting is accelerating at an ever-increasing rate. It’s exciting times, however, we need to be careful to temper our expectations of this technology with the realities. The human body is incredibly complex, and trying to replicate the many things that it does is difficult, challenging work.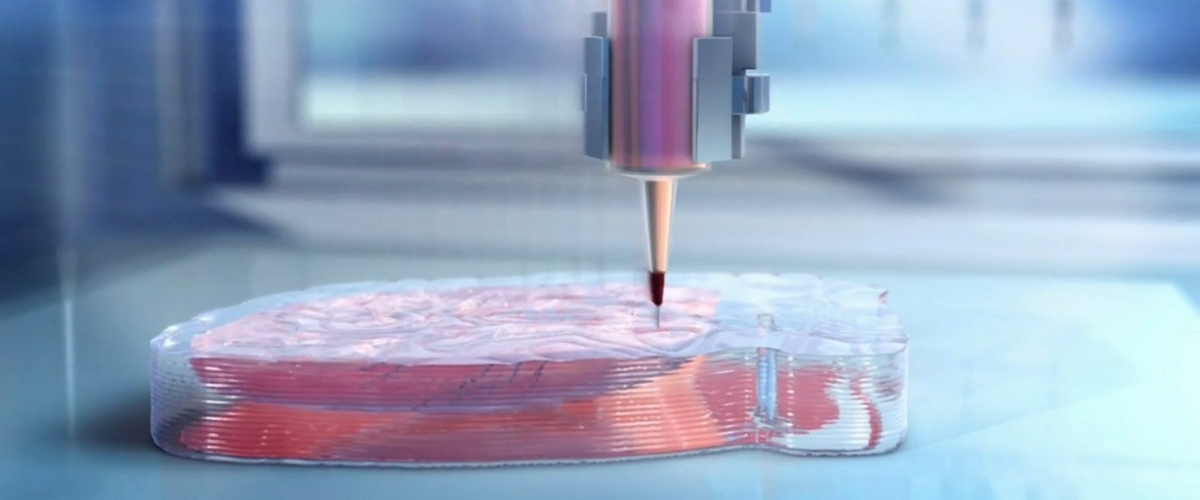 Those working in the field are making advances every day, in both the technology and in their understanding of how it can be used and improved. While we’re not quite there yet, there is no doubt that the future of medicine will be very different with bioprinting involved.
Those working in the field are making advances every day, in both the technology and in their understanding of how it can be used and improved. While we’re not quite there yet, there is no doubt that the future of medicine will be very different with bioprinting involved.
all about printing organs on a 3D printer
Printing organs on a 3D printer or bioprinting is a promising technology for growing healthy and living organs to replace damaged or missing ones. In addition to a 3D printer, bioprinting requires a model of an organ, patient cell material, and an environment where the organ will remain until implantation.
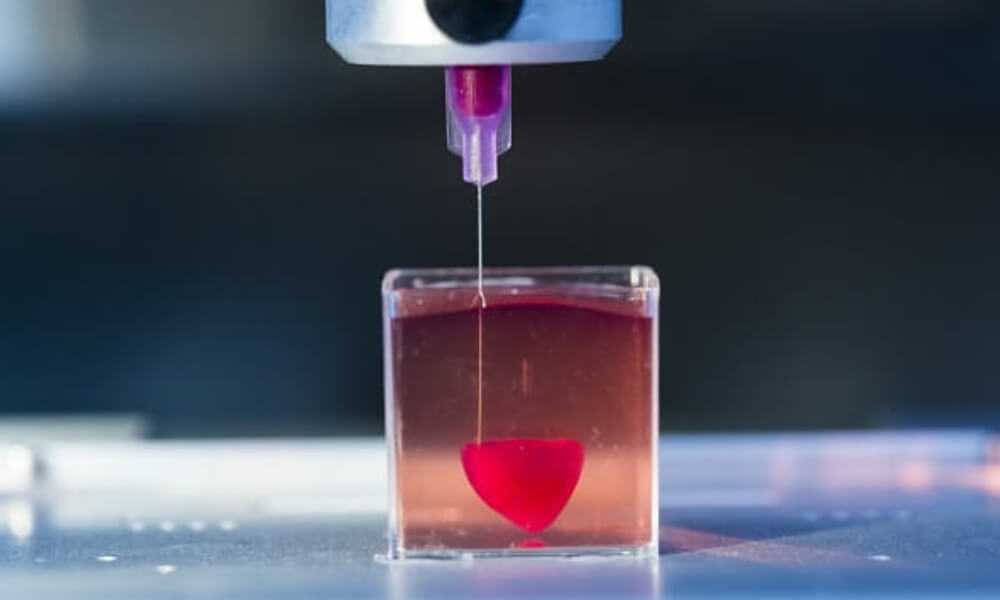
Printed organs are better than prostheses and transplanted body parts. Their capabilities are identical to native ones and they are not rejected by the immune system if they are created from the patient's DNA. Bioprinting will reduce the time to obtain the desired organ and save the lives of patients who need an immediate transplant.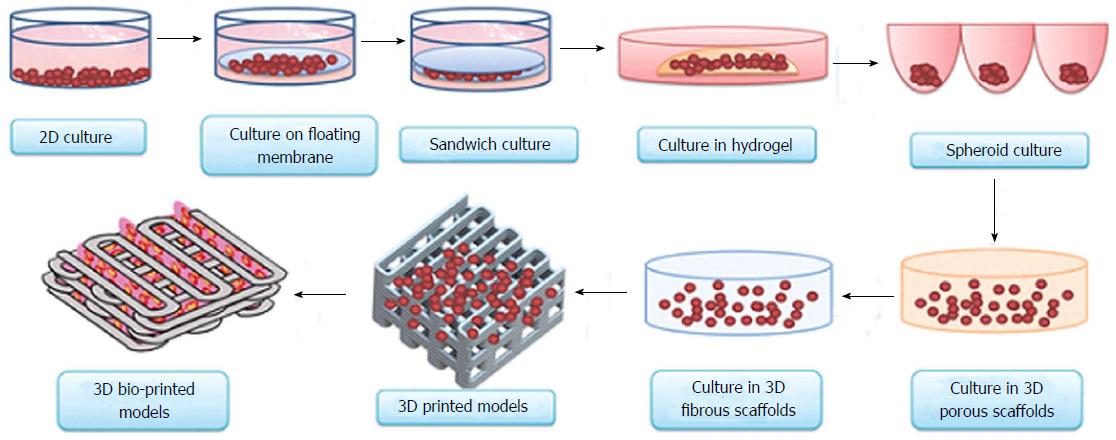
Printing organs on a 3D printer has already been successfully tested on animals. Scientists at Northwestern University implanted artificial ovaries in sterilized mice and they gave birth to healthy mice. In the Chinese company Sichuan Revotek, rhesus monkeys have been implanted with blood vessels grown from the material of the same monkeys.
From human body parts, only internal tissues and skin are printed so far. Reduced but working copies of ears and noses are created. The first printing of human organs is expected by 2030.
How bioprinting works
Research groups or companies are developing different bioprinting concepts:
- Wireframe. The growth of living cells on an inorganic base, which disappears with the development of natural connections between cells. The main difficulty is to find a material that is as elastic or rigid as the organ being replaced. It must degrade quickly so as not to interfere with the strengthening of the extracellular matrix and dissolve without leaving toxic compounds.
 Hydrogel, titanium, gelatin, synthetic and biopolymers are suitable for wireframe printing.
Hydrogel, titanium, gelatin, synthetic and biopolymers are suitable for wireframe printing.
- Frameless. Application of prepared cells on a hydrogel base. While the cells are in the printer, they are cooled and are in thin hydrogel spheroids. When printing, the temperature rises to 36.6°C, the spheroids scatter, and the cells gradually form their own natural framework - the cellular matrix. This printing is less common than wireframe printing - it appeared later and is more difficult to reproduce.
- Mimicry. The technology of the future, involves the creation of complete copies of organs at once. For it, bioprinting is being developed at the molecular level and in-depth studies of the nature of cells are being carried out.
Methods for 3D printing of organs
Inkjet. The first devices for bioprinting were inkjet, conventional printers also use this method. They store biological material in cartridges that are sprayed onto a hydrogel substrate like paint on paper. Disadvantages - inaccurate droplet ejection and blockage of the spray nozzle with possible death of cellular material. Inkjet printing of organs on a printer is not suitable for viscous materials because they are not atomized. The scope is limited to the restoration of bone, cartilage, muscles and skin. Advantages - low cost and mass reproducibility.
Disadvantages - inaccurate droplet ejection and blockage of the spray nozzle with possible death of cellular material. Inkjet printing of organs on a printer is not suitable for viscous materials because they are not atomized. The scope is limited to the restoration of bone, cartilage, muscles and skin. Advantages - low cost and mass reproducibility.
Microextrusion. This method is used in inorganic 3D printing. For printing, a pneumatic supply of material is used in a movable extruder head, which stacks the cells. The more heads, the more accurate and faster the printer. Disadvantages - the denser the cells fit, the less they survive. With a comparable stacking density, more cells die from microextrusion printing than from inkjet printing. Advantages - suitable for 3D printing of high-density organs, fine-tuning of the material supply due to pressure regulation.
Laser. Common in industry but used in bioprinting. A laser is used to heat glass with a liquid cell substrate. At the beam concentration point, excess pressure is created, which pushes the cells to the desired area of the substrate. A reflective element is placed between the beam and the glass with biomaterial, which reduces the power of the beam. Disadvantages - increased metal content in the cells from the evaporation of the reflective element. Price. Advantages - controlled up to individual cells, laying of biomaterial.
At the beam concentration point, excess pressure is created, which pushes the cells to the desired area of the substrate. A reflective element is placed between the beam and the glass with biomaterial, which reduces the power of the beam. Disadvantages - increased metal content in the cells from the evaporation of the reflective element. Price. Advantages - controlled up to individual cells, laying of biomaterial.
Who offers 3D printing of organs
Bioprinting companies that offer 3D printing of organs or sell bioprinters:
- Organovo - San Diego, USA. Prints and sells liver tissue " exVive3D" to pharmaceutical companies. In 2009, Organovo, together with the Austrian Invetech, launched the first mass-produced bioprinter, Novogen.
- BioBots is a startup that presented a cheap commercial bioprinter at TechCrunch 2013. Today, the Biobot 1 model is available for purchase, Biobot 2 is still in development, but already presented on the company's website.

- 3D Bioprinting Solutions - Russia , Moscow. Focused on frameless printing, has developed its FABION 3D printer and is working on its own organoprinting technology
- Cyfuse Biomedical - Tokyo, Japan. They developed the Regenovo bioprinter, which was used to print skin and successfully grew 2-mm vessels.
How much does a 3D bioprinter cost
The average cost of a bioprinter is a quarter of a million dollars, but budget models are available for up to $10,000. Most printers available for purchase are extrusion type and work with frame printing.
- 3D Bioplotter - $200,000 Envision TEC, Germany.
- Novogen MMX - $250.00. Organovo, USA.
- Biobot 1 - $10,000. Biobots, USA.
- 3DDiscovery - $200,000. RegenHU & Biofactory, Switzerland.
- BioAssemblyBot - $160,000 Advanced Solutions, The Netherlands.
Supporting a patient with life support devices costs about $75,000 per year. In 10 years, the patient will spend $1 million. The printer costs $200,000 and the operation costs about the same. Considering how much it costs to print organs, the operation using 3D bioprinting is reduced by 50%.
In 10 years, the patient will spend $1 million. The printer costs $200,000 and the operation costs about the same. Considering how much it costs to print organs, the operation using 3D bioprinting is reduced by 50%.
The Future of Bioprinting
3D bioprinting has gone from concept to working and commercially successful technology. So far, the main clients of bioprinting companies are large pharmaceutical corporations. They speed up drug testing by testing them directly on printed human tissues.
Expensive bioprinters won't be in city clinics in 5 years, but some patients are already recovering thanks to 3D printing. The jaw of an 83-year-old woman from Belgium was struck by osteomyelitis. The restoration was more expensive and would have taken longer than the removal of the diseased jaw and the implantation of a printed new one. A team of doctors led by Professor Jules Poukan performed the operation and the woman was able to speak immediately after the operation. The development of bioprinting will lead to medical practices where it is easier to remove an injured limb and grow a new one than to treat injuries that are now treated without amputation.
The development of bioprinting will lead to medical practices where it is easier to remove an injured limb and grow a new one than to treat injuries that are now treated without amputation.
Medicine of the distant future minimizes mechanical intervention in the body. The scalpel will remain in the past - a swarm of nanorobots will print organs immediately inside the body. In 2018, a full-fledged printing of a human organ on a printer is planned - the kidneys. Then the bronchi, arteries and heart will be printed. But even clinical trials on humans are about 10 years away, and mass 3D printing of human organs and body parts will come even later.
In addition to doctors, bioprinting is attractive to cosmetologists and plastic surgeons. The desire to remain young and beautiful, and not the treatment of rare and complex diseases, will make 3D printing of human organs mass. Perhaps the people of the future will change organs and appearance as easily as smartphones.
Organ printing: how 3D bioprinting technology has advanced and what is preventing its development In research centers and hospitals around the world, advances in 3D printing and bioprinting are providing new opportunities for human treatment and scientific research.
 In the coming decades, bioprinting could be the next major milestone in healthcare and personalized medicine.
In the coming decades, bioprinting could be the next major milestone in healthcare and personalized medicine. Let's talk about bioprinting technology, the latest advances in the industry and the limitations that professionals face.
How a 3D printer works
Traditional printers, like the one you have at home or office, work in two dimensions. They can print text or images on a flat surface (usually paper) using the x (horizontal) and y (vertical) dimensions. 3D printers add another dimension - depth (z). During the printing process, the printer heads can move up and down, left and right, back and forth, but instead of delivering ink to paper, they distribute various materials - polymers, metal, ceramics and even chocolate - until the "print" of a holistic, voluminous object , layer by layer in a process known as "additive manufacturing".
To create a 3D object, you need a plan for it, a digital file created with modeling software. After its creation, the computer-generated model is sent to the printer. Your chosen material is loaded into the machine and ready to be heated to easily flow out of the printer nozzle. As the printer reads the plan, its head moves, depositing successive layers of the selected material to create the final product.
After its creation, the computer-generated model is sent to the printer. Your chosen material is loaded into the machine and ready to be heated to easily flow out of the printer nozzle. As the printer reads the plan, its head moves, depositing successive layers of the selected material to create the final product.
As each layer is printed, it is solidified either by cooling or by mixing two different solutions delivered by the printer head. The new layers precisely lay down on the previous ones to make a stable, cohesive element. In this way, you can create almost any shape, including a moving one.
3D printing allows you to create objects with geometric structures that would be difficult or impossible to make in other ways. A wide range of products are already being created using 3D printers, including jewelry, clothing, toys, and high-end industrial products. Even a 10-year-old Moscow schoolboy has learned how to work with a 3D printer: he prints 3D figures to order and sells them through Instagram.
How a bioprinter works
Bioprinters work in much the same way as 3D printers, with one key difference - they deposit layers of biomaterial, which can include living cells, to create complex structures such as blood vessels or skin tissue.
Living cells? Where do they get them? Every tissue in the body is made up of different types of cells. The required cells (kidney, skin, and so on) are taken from the patient and then cultured until there are enough of them to create "bio-ink" that is loaded into the printer. This is not always possible, therefore, for some tissues, stem cells are taken that are capable of becoming any cell in the body (organism), or, for example, porcine collagen protein, seaweed and others.
Often used in bioprinting is chitosan, a polysaccharide obtained from the external skeleton of mollusks (eg shrimp) or by fermenting fungi. This material has high biocompatibility and antibacterial properties. Its disadvantage is the low rate of gelation. Another popular material is a polysaccharide isolated from seaweed called agarose. Its advantages are high stability and the possibility of non-toxic cross-linking during research. However, this biomaterial does not decompose and has poor cell adhesion (the ability of cells to stick together with each other and with other substrates).
Its disadvantage is the low rate of gelation. Another popular material is a polysaccharide isolated from seaweed called agarose. Its advantages are high stability and the possibility of non-toxic cross-linking during research. However, this biomaterial does not decompose and has poor cell adhesion (the ability of cells to stick together with each other and with other substrates).
Collagen, the primary structural protein found in the skin and other connective tissues, is of high biological importance. It is the most abundant protein in mammals and a major component of connective tissue. Its disadvantages for bioprinting include the property of acid solubility. More information about biomaterials can be found here.
Based on computer designs and models, often scans and MRIs taken directly from the patient, the printer heads place the cells exactly where they are needed, and within a few hours an organic object is built from a large number of very thin layers.
Organovo bioprinter creates tissues that mimic the structure and composition of various human organs
Source: Pbs. org
org
Scaffolding for ear or nose replacement at Wake Forest University in Winston-Salem, North Carolina
Source: CBS News
Computer displays an image of a "scaffolding" for the human ear, created in a laboratory at Wake Forest University in Winston-Salem, North Carolina
Source: CBS News
Usually more than just cells are needed, so most bioprinters also supply some kind of organic or synthetic "glue" - a soluble gel or collagen scaffold to which cells can attach and grow. This helps them form and stabilize in the correct shape. Surprisingly, some cells can take the correct position on their own without any "scaffolding". How do they know where to go? How do embryonic cells develop in the uterus, or does adult tissue move to repair damage? Same here.
Universities, researchers and private companies around the world are involved in the development of bioprinting technologies. Let's take a look at some of the amazing things they are working on.
Bioprinting in Russia
3D Bioprinting Solutions is a biotechnology research laboratory founded by medical company INVITRO. The activity of the laboratory is the development and production of bioprinters and materials in the field of three-dimensional bioprinting and scientific research. August 23, 20193D Bioprinting Solutions laboratory sent a new batch of cuvettes to the ISS to continue experiments on bioprinting in space, which began in 2018. This was reported in the press center of the laboratory. This time it is planned to use organic and inorganic components to assemble bone tissue on the world's first space bioprinter Organ.Aut.
Symposium "Biofabrication in space"
Source: Zdrav.Expert
Organ.Aut magnetic bioprinter
Source: Zdrav.Expert
The astronauts will also grow protein crystals and experiment with printing biofilms of bacteria to study their behavior in zero gravity. Russian scientists expect to receive unique scientific data that can be applied in the development of new drugs.
Scientific director of 3D Bioprinting Solutions and leading researcher of the Institute of Regenerative Medicine, Candidate of Medical Sciences Vladimir Mironov, in his speech at the Department of Anatomy of Sechenov University on September 2, noted: “Living cells, tissues and human organs will be synthesized already in the current century. To do this, morphological sciences, such as microscopic anatomy and histology, must be digitized or digitalized, that is, digitized and made available for computer programs of robotic bioprinters, since without digital models it is impossible to print human tissues and organs.”
Bioprinting around the world
Every year, millions of people around the world need a bone transplant. Modern bone grafts often use cement-based synthetic material in combination with the patient's own bone. However, the use of these materials has a number of limitations - some transplants caused rejection and inflammatory processes in patients. Reproduction of the natural bone-cartilage "interface" has also been problematic.
Reproduction of the natural bone-cartilage "interface" has also been problematic.
However, a team from Swansea University in 2014 developed a bioprinting technology that allows the creation of an artificial bone prosthesis in the exact shape of the desired bone, using a biocompatible material that is both durable and regenerative. At the same time, scientists from the University of Nottingham in England were working on similar studies.
It takes about two hours to print a small bone. Therefore, surgeons can do it right in the operating room. This part of the bone is then covered with adult stem cells that can develop into almost any other type of cell. This is combined with bio-ink from the printer, a combination of polylactic acid (which provides mechanical strength to bone) and alginate, a gel-like substance that serves as a shock-absorbing material for cells. The end product is then implanted into the body, where it will completely disappear within about three months and be replaced by new bone.
Researchers hope that in the future, bioprinted bones can be created with sufficient reliability to support complex spinal reconstruction, and that the bone material will be further improved to increase its compatibility with cartilage cells.
Source: ETH Zurich
Successful 3D printing of human cartilage may soon completely replace artificial implants for people in need of reconstructive surgery. Back in 2015, scientists in Zurich developed technology that would allow hospitals to print a full-size human nose implant in less than 20 minutes. They believe that any cartilage implant can be made using their technique.
Researcher Matti Kesti described the technology as follows:
“
“A serious car accident can cause the driver or passenger to suffer complex nose injuries. The nose can be restored by creating a 3D model on a computer. At the same time, a biopsy of the patient is performed and cartilage cells are removed from the victim's body, such as from a knee, a finger, an ear, or fragments of a broken nose. The cells are spawned in the laboratory and mixed with the biopolymer. From this suspension, a model of nasal cartilage is created using a bioprinter, which is implanted into the patient during surgery. In the process, the biopolymer is used simply as a mold. It is subsequently broken down by the body's own cartilage cells. And in a couple of months it will be impossible to distinguish between the graft and the person’s own nasal cartilage.”
The cells are spawned in the laboratory and mixed with the biopolymer. From this suspension, a model of nasal cartilage is created using a bioprinter, which is implanted into the patient during surgery. In the process, the biopolymer is used simply as a mold. It is subsequently broken down by the body's own cartilage cells. And in a couple of months it will be impossible to distinguish between the graft and the person’s own nasal cartilage.”
Matti Kesti
Since the implant was grown from the body's own cells, the risk of rejection will be much lower than for an implant made of, say, silicone. An additional advantage is that the bioimplant grows with the patient, which is especially important for children and young adults.
If a person is severely burned, healthy skin can be taken from another part of the body and used to cover the affected area. Sometimes intact skin is missing.
Researchers at Wake Forest School of Medicine have successfully designed, built and tested a printer that can print skin cells directly onto a burn wound. The scanner very accurately determines the size and depth of damage. This information is sent to a printer and skin is printed to cover the wound. Unlike traditional skin grafts, it only takes a patch of skin one-tenth the size of a burn to grow enough cells to print. While this technology is still in the experimental stage, the researchers hope that it will be widely available within the next five years.
The scanner very accurately determines the size and depth of damage. This information is sent to a printer and skin is printed to cover the wound. Unlike traditional skin grafts, it only takes a patch of skin one-tenth the size of a burn to grow enough cells to print. While this technology is still in the experimental stage, the researchers hope that it will be widely available within the next five years.
As already mentioned, 3D printers print products in layers, and since the skin is a multi-layered organ with different types of cells, it is well suited for this type of technology. However, researchers still have a lot of problems to solve, in particular, how to prevent damage to cells from the heat generated by the printer. And of course, like most parts of the human body, the skin is more complex than it first appears—there are nerve endings, blood vessels, and a host of other aspects to consider.
Blood vessels
Biomechanical engineer Monica Moya holding a Petri dish with printed alginate-based biotubes. Biotubes can act as temporary blood vessels similar to blood vessels that help create a patch of living tissue.
Biotubes can act as temporary blood vessels similar to blood vessels that help create a patch of living tissue.
Source: embodi3D
With tens of thousands of miles of veins, arteries and capillaries in the human body, researchers are working to replace them if they ever wear out. The creation of viable blood vessels is also essential for the proper functioning of all other potential bioprinted body parts.
Biomechanical Engineer Monica Moya of Livermore National Laboratory. Lawrence uses bioprinting to create blood vessels. The materials created by her bioprinters are engineered to allow small blood vessels to develop on their own.
This development takes time, so vials of cells and other biomaterials are printed to help deliver vital nutrients to the printed environment. After a while, self-assembled capillaries connect with bioprinted tubes and begin to deliver nutrients to cells on their own, mimicking the work of these structures in the human body.
Internal organs
Many researchers hope that in 20 years the lists of patients waiting for organ transplants will become a thing of the past. They envision a world where any organ can be printed and transplanted in just a few hours, without rejection or complications, because these organs will be created from body cells according to the individual characteristics of each patient. Currently, bioprinting of fully functional complex internal organs is not possible, but research is ongoing (and not without success).
Bladder
For example, the bladder is already printed. In 2013, at Wake Forest University in the US, researchers successfully took cells from a patient's original, poorly functioning bladder, cultured them, and added additional nutrients. The 3D shape of the patient's bladder was then printed and the cultured cells soaked through it. The form was placed in an incubator and, when it reached the desired condition, it was transplanted into the patient's body.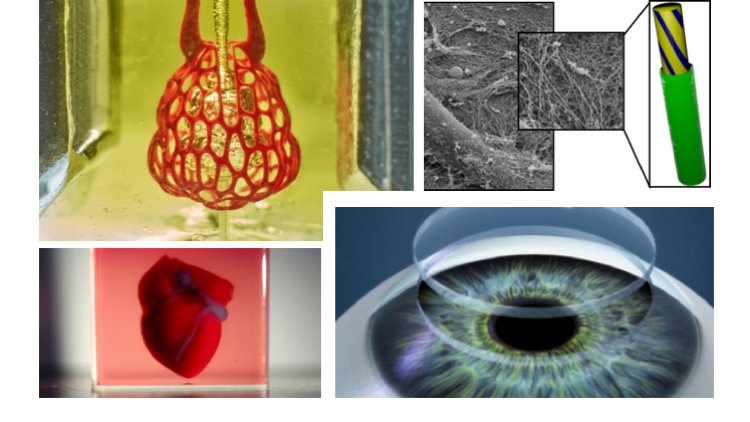 The mold will eventually collapse, leaving only the organic material. The same team successfully created viable urethras.
The mold will eventually collapse, leaving only the organic material. The same team successfully created viable urethras.
Physicians and scientists at the Wake Forest Institute for Regenerative Medicine (WFIRM) were the first in the world to create laboratory-grown organs and tissues that were successfully transplanted into humans. Right now they are working on growing tissues and organs for more than 30 different areas of the body, from the kidneys and trachea to cartilage and lungs. They also aim to accelerate the availability of these treatments to patients.
Scientists in Australia are doing similar research as well. They used human stem cells to grow a kidney organ that contains all the necessary cell types for a kidney. Such cells can serve as a valuable initial source for bioprinting more complex kidney structures.
MD, Professor of Urology, Professor of the Institute of Regenerative Medicine Anthony Atala shows a kidney created by a bioprinter. A modified desktop inkjet printer sprays cells instead of ink. The cells were cultured from the patient and the structural template for the kidney was obtained from the MRI (so it is the correct size and shape).
A modified desktop inkjet printer sprays cells instead of ink. The cells were cultured from the patient and the structural template for the kidney was obtained from the MRI (so it is the correct size and shape).
Using this technology, back in 2001, Atala printed and successfully transplanted a bladder into a young man, Jake.
Source: TedEd
Heart
Heart cells, laboratory-grown organelles. Source
Surprisingly, it is the human heart that can become one of the easiest organs to print, since, in fact, it is a pump with tubes. Of course, everything is not so simple, but many researchers believe that humanity will learn to print hearts before kidneys or liver.
Researchers at the Wake Forest Institute for Regenerative Medicine in April 2015 created "organoids" - 3D printed fully functional, beating heart cells.
In April 2019, Israeli scientists printed the world's first 3D heart. It is still very small, the size of a cherry, but it is able to perform its functions. The 3D heart with blood vessels uses personalized "ink" of collagen, a protein that supports cell structures, and other biological molecules.
The 3D heart with blood vessels uses personalized "ink" of collagen, a protein that supports cell structures, and other biological molecules.
A Tel Aviv University researcher holds the world's first 3D printed heart on April 15, 2019.
Source: Haaretz
“This is the first time anyone anywhere has successfully designed and printed a whole heart with cells, blood vessels, ventricles and chambers,” said Tel Aviv University scientist Professor Tal Dvir.
So far, scientists have been able to print tissue from cartilage and the aortic valve, for example, but the challenge has been to create tissue with vascularity—the blood vessels, including capillaries, without which organs cannot survive, let alone function.
The Tel Aviv scientists started with human adipose tissue and separated the cellular and non-cellular components. They then reprogrammed the cells to become undifferentiated stem cells, which could then become cardiac or endothelial.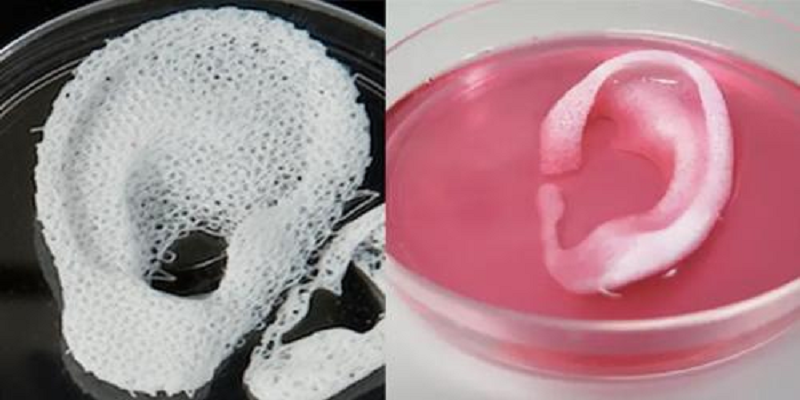 Endothelium - a single layer of flat cells lining the inner surface of the heart cavities, blood and lymphatic vessels. Endothelial cells perform many functions of the vascular system, such as controlling blood pressure, regulating the components of blood clotting, and the formation of new blood vessels.
Endothelium - a single layer of flat cells lining the inner surface of the heart cavities, blood and lymphatic vessels. Endothelial cells perform many functions of the vascular system, such as controlling blood pressure, regulating the components of blood clotting, and the formation of new blood vessels.
Non-cellular materials, including large amounts of proteins, were processed into a "personalized hydrogel" that served as "printing ink".
It will be years before this technology can create organs for efficient transplantation. However, the achievements of scientists in Tel Aviv are a huge milestone along the way.
Medical research and pharmacology
One of the key potential uses for bioprinted living materials is in the field of medical and drug research. Bioprinted tissues have several cell types with different densities and key architectural features. This allows researchers to study the impact of various diseases on the body, the stages of disease progression and possible treatments in the natural microenvironment.
One of the most impressive developments in recent years is the development of a desktop brain at the ARC Center of Excellence in 2016. The researchers were able to use a 3D printer to create a 3D printed six-layer structure that includes nerve cells that mimic the structure of brain tissue.
This opens up huge potential benefits for researchers, pharmaceuticals and private companies, because it will allow them to test new products and drugs on tissue that accurately reflects the responses of human brain tissue, as opposed to animal samples, which may cause a completely different response. The desktop brain can also be used to further investigate diseases such as schizophrenia or Alzheimer's.
We are far from printing the brain, but the ability to arrange cells to form neural networks is a significant step forward. By allowing researchers to work with human tissue in real time, testing processes can be greatly accelerated and results can be more realistic and accurate. It will also reduce the need to use laboratory animals for medical tests and potentially dangerous human testing.
It will also reduce the need to use laboratory animals for medical tests and potentially dangerous human testing.
Medical simulators and data registries
Source: Simbionix
About 3,000 medical simulators are currently in use around the world to help doctors practice complex procedures. Virtual blood vessels, 3D printed organs... and no animal suffers!
The American company 3D Systems has created an industry segment called VSP (Virtual Surgical Planning). This approach to personalized surgery combines expertise in medical imaging, surgical simulation and 3D printing. Surgeons using the Simbionix medical simulator for the first time often report feeling physical pain while empathizing with their virtual patient - the experience is so realistic. Organs and tissues look completely real. When stitching an organ, the surgeon sees on the screen a needle that enters the tissue, and pulls the thread. If the doctor does something wrong, the virtual blood vessels break and the organ begins to bleed. These simulators were developed by the Israeli company Symbionix, which was acquired by 3D Systems in 2014.
These simulators were developed by the Israeli company Symbionix, which was acquired by 3D Systems in 2014.
On September 3, 2019, the Radiology Society of North America (RSNA) and the American College of Radiology (ACR) announced the launch of a new 3D Medical Printing Clinical Data Registry to collect data on treatment outcomes using 3D printing at the point of care. This information will be a powerful tool to assess and improve patient care in real time, drive ongoing research and development, and inform patients and healthcare professionals about the best course of care.
“
“The creation of a joint RSNA-ACR 3D printing registry is essential to the advancement of clinical 3D printing. The registry will collect data to support the appropriate use of this technology and its implications for clinical decision making.”
William Widock, Professor of Radiology at the University of Michigan and Chairman of the RSNA 3D Printing Special Interest Group (SIG)
According to RSNA, the information in the registry will allow for the necessary analysis to demonstrate the clinical value of 3D printing. Due to the wide variety of clinical indications, different technologies for creating physical models from medical images, and the complexity of the models, it is problematic to choose the optimal treatment method. The registry will help solve this problem.
Due to the wide variety of clinical indications, different technologies for creating physical models from medical images, and the complexity of the models, it is problematic to choose the optimal treatment method. The registry will help solve this problem.
Bioprinting software
Bioprinter and bioprinting software manufacturer Allevi introduced Allevi Bioprint Pro software on September 5, 2019. Built-in model generation and integrated slicing will allow you to focus more on experimenting, rather than setting up the printer. The program runs entirely in the cloud, which means you can create your biostructures, define materials, and track prints right from a web browser on any computer.
According to the development team, the new bioprinter with the above software is powerful and easy to use and represents another piece of the puzzle on the way to 3D printed organs.
At the same time, CELLINK, the first bio-ink company, announced the launch of a new product to become the most flexible bio-printing platform on the market. The BIO X6 bioprinter, which has no analogues at the moment, has the ability to combine more bioprinting materials, cells and tools.
The BIO X6 bioprinter, which has no analogues at the moment, has the ability to combine more bioprinting materials, cells and tools.
Why is this taking so long?
Complex body structure
The human body and its various components are much more complex than a plastic toy. The human organ has a complex network of cells, tissues, nerves, and structures that must be arranged in specific ways to function properly. From placing thousands of tiny capillaries in the liver to actually getting a printed heart that "beats" and contracts in the human body, there is still a lot of research and testing.
Legal regulation
In addition, bioprinting technologies, like all new medical treatments, must pass safety tests and due process of regulation before they become available.
Special software and hardware
It also takes time to develop special software and hardware. These programs can be written only with the appropriate data (medical, clinical, statistical, mathematical, and so on), which someone must first collect, analyze, systematize and digitize.
Working through all of these steps requires the integration of technologies from various fields, including engineering, biomaterials science, cell biology, physics, mathematics and medicine. So we need to be a little more patient.
The main thing is to know that those who work in the field, doctors and engineers, programmers and scientists are making progress every day both in the bioprinting technology itself and in understanding how it can be used and improved. Although we are not quite there yet, there is no doubt that medicine will be very different in 10-20 years, thanks also to bioprinting.
In brief
Bioprinting is an extension of traditional 3D printing.
Bioprinting can produce living tissue, bones, blood vessels, and possibly entire organs for use in medical procedures, medical training, and testing.
The cellular complexity of a living organism has made 3D bioprinting slower to develop than conventional 3D printing.