Biocompatible 3d printer filament
A Low-Cost Method to Prepare Biocompatible Filaments with Enhanced Physico-Mechanical Properties for FDM 3D Printing
Save citation to file
Format: Summary (text)PubMedPMIDAbstract (text)CSV
Add to Collections
- Create a new collection
- Add to an existing collection
Name your collection:
Name must be less than 100 characters
Choose a collection:
Unable to load your collection due to an error
Please try again
Add to My Bibliography
- My Bibliography
Unable to load your delegates due to an error
Please try again
Your saved search
Name of saved search:
Search terms:
Test search terms
Email: (change)
Which day? The first SundayThe first MondayThe first TuesdayThe first WednesdayThe first ThursdayThe first FridayThe first SaturdayThe first dayThe first weekday
Which day? SundayMondayTuesdayWednesdayThursdayFridaySaturday
Report format: SummarySummary (text)AbstractAbstract (text)PubMed
Send at most: 1 item5 items10 items20 items50 items100 items200 items
Send even when there aren't any new results
Optional text in email:
Create a file for external citation management software
. 2021;18(6):700-711.
doi: 10.2174/1567201817999201103195456.
Deck Khong Tan 1 , Niko Münzenrieder 2 , Mohammed Maniruzzaman 3 , Ali Nokhodchi 1
Affiliations
Affiliations
- 1 Pharmaceutics Research Laboratory, Department of Chemistry, School of Life Sciences, University of Sussex, Brighton BN1 9QJ, United Kingdom.
- 2 Faculty of Science and Technology, Free University of Bozen-Bolzano, Bozen, Italy.
- 3 Pharmaceutical Engineering and 3D Printing (PharmE3D) Lab, Division of Molecular Pharmaceutics and Drug Delivery College of Pharmacy, University of Texas, Austin, TX 78712, United States.

- PMID: 33155909
- DOI: 10.2174/1567201817999201103195456
Deck Khong Tan et al. Curr Drug Deliv. 2021.
. 2021;18(6):700-711.
doi: 10.2174/1567201817999201103195456.
Authors
Deck Khong Tan 1 , Niko Münzenrieder 2 , Mohammed Maniruzzaman 3 , Ali Nokhodchi 1
Affiliations
- 1 Pharmaceutics Research Laboratory, Department of Chemistry, School of Life Sciences, University of Sussex, Brighton BN1 9QJ, United Kingdom.

- 2 Faculty of Science and Technology, Free University of Bozen-Bolzano, Bozen, Italy.
- 3 Pharmaceutical Engineering and 3D Printing (PharmE3D) Lab, Division of Molecular Pharmaceutics and Drug Delivery College of Pharmacy, University of Texas, Austin, TX 78712, United States.
- PMID: 33155909
- DOI: 10.2174/1567201817999201103195456
Abstract
Background: Fused Deposition Modelling (FDM) 3D printing has received much interest as a fabrication method in the medical and pharmaceutical industry due to its accessibility and cost-effectiveness. A low-cost method to produce biocompatible and biodegradable filaments can improve the usability of FDM 3D printing for biomedical applications.
A low-cost method to produce biocompatible and biodegradable filaments can improve the usability of FDM 3D printing for biomedical applications.
Objectives: The feasibility of producing low-cost filaments suitable for FDM 3D printing via single screw and twin-screw hot melt extrusion was explored.
Methods: A single-screw extruder and a twin-screw extruder were used to produce biocompatible filaments composed of varying concentrations of polyethylene glycol (PEG) at 10%, 20%, 30% w/w and polylactic acid (PLA) 90%, 80% and 70% w/w, respectively. DSC, TGA and FTIR were employed to investigate the effect of PEG on the PLA filaments.
Results: The presence of PEG lowered the processing temperature of the formulation compositions via melt-extrusion, making it suitable for pharmaceutical applications. The use of PEG can lower the melting point of the PLA polymer to 170°C, hence lowering the printing temperature. PEG can also improve the plasticity of the filaments, as the rupture strain of twin-screw extruded filaments increased up to 10-fold as compared to the commercial filaments. Advanced application of FTIR analysis confirmed the compatibility and miscibility of PEG with PLA.
The use of PEG can lower the melting point of the PLA polymer to 170°C, hence lowering the printing temperature. PEG can also improve the plasticity of the filaments, as the rupture strain of twin-screw extruded filaments increased up to 10-fold as compared to the commercial filaments. Advanced application of FTIR analysis confirmed the compatibility and miscibility of PEG with PLA.
Conclusion: Twin-screw extrusion is more effective in producing a polymeric mixture of filaments as the mixing is more homogenous. The PEG/PLA filament is suitable to be used in 3D printing of medical or pharmaceutical applications such as medical implants, drug delivery systems, or personalised tablets.
Keywords: Biocompatible; FDM 3D printing; biopolymers; filaments; hot melt extrusion; polyethylene glycol (PEG)..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience. net.
net.
Similar articles
-
Role of release modifiers to modulate drug release from fused deposition modelling (FDM) 3D printed tablets.
Shi K, Salvage JP, Maniruzzaman M, Nokhodchi A. Shi K, et al. Int J Pharm. 2021 Mar 15;597:120315. doi: 10.1016/j.ijpharm.2021.120315. Epub 2021 Feb 1. Int J Pharm. 2021. PMID: 33540000
-
Development of immediate release (IR) 3D-printed oral dosage forms with focus on industrial relevance.
Fanous M, Gold S, Hirsch S, Ogorka J, Imanidis G. Fanous M, et al. Eur J Pharm Sci. 2020 Dec 1;155:105558. doi: 10.1016/j.ejps.2020.105558. Epub 2020 Sep 16. Eur J Pharm Sci. 2020. PMID: 32946957
-
Preparation and characterization of hot-melt extruded polycaprolactone-based filaments intended for 3D-printing of tablets.
Viidik L, Vesala J, Laitinen R, Korhonen O, Ketolainen J, Aruväli J, Kirsimäe K, Kogermann K, Heinämäki J, Laidmäe I, Ervasti T. Viidik L, et al. Eur J Pharm Sci. 2021 Mar 1;158:105619. doi: 10.1016/j.ejps.2020.105619. Epub 2020 Oct 25. Eur J Pharm Sci. 2021. PMID: 33115676
-
Advanced Pharmaceutical Applications of Hot-Melt Extrusion Coupled with Fused Deposition Modelling (FDM) 3D Printing for Personalised Drug Delivery.
Tan DK, Maniruzzaman M, Nokhodchi A. Tan DK, et al. Pharmaceutics. 2018 Oct 24;10(4):203. doi: 10.3390/pharmaceutics10040203. Pharmaceutics. 2018. PMID: 30356002 Free PMC article. Review.
-
Hot Melt Extrusion and its Application in 3D Printing of Pharmaceuticals.
Deshkar S, Rathi M, Zambad S, Gandhi K.
 Deshkar S, et al. Curr Drug Deliv. 2021;18(4):387-407. doi: 10.2174/1567201817999201110193655. Curr Drug Deliv. 2021. PMID: 33176646 Review.
Deshkar S, et al. Curr Drug Deliv. 2021;18(4):387-407. doi: 10.2174/1567201817999201110193655. Curr Drug Deliv. 2021. PMID: 33176646 Review.
See all similar articles
Cited by
-
Low-Cost Cranioplasty-A Systematic Review of 3D Printing in Medicine.
Czyżewski W, Jachimczyk J, Hoffman Z, Szymoniuk M, Litak J, Maciejewski M, Kura K, Rola R, Torres K. Czyżewski W, et al. Materials (Basel). 2022 Jul 6;15(14):4731. doi: 10.3390/ma15144731. Materials (Basel). 2022. PMID: 35888198 Free PMC article. Review.
MeSH terms
Substances
Full text links
Bentham Science Publishers Ltd.
Cite
Format: AMA APA MLA NLM
Send To
FibreTuff - 3D Printing, Biocompatible, Bone
FibreTuff medical grade filament for producing anatomical bone like analogs
The 3D printing FibreTuff biocompatible materials in a filament have a bone like surface texture, as well as appearance similar to cadaver models - skulls, femurs, calcaneus, talus, metatarsal, skull cap, mandible, sacrum.
Printed micropores with FibreTuff
FibreTuff biocompatible filament has been printed in a micropore cellular structure. The 3D printing of the micropore structure has been exposed to simulated body fluids without pore closure. Write [email protected] for more information.
Write [email protected] for more information.
Annealing FibreTuff for increased performance and better printing
The 3D printing of FibreTuff medical grade filament can be annealed to increase physical performance in some cases beyond 10%. Please desiccant dry FibreTuff filaments for 8 hrs min. before printing. The oven temperatures of 225F / 127C up to 2 hr will help increase flexibility and strength.
Mechanicals of FibreTuff
The FibreTuff I has proven to be biocompatible with similar properties to Nylon 11 or 12. The specific gravity of FibreTuff I is 1.05 g/cc. This filament for 3D Printing is user friendly and doesn't require extensive drying like other highly engineered resins. The FibreTuff is anisotropic, prints are stronger in the Z direction versus X and Y.
FibreTuff medical grade filament produced anatomical bone like models that perform like real bone
The 3D printed hand models produced at VosFox medical with FibreTuff showed details having 27 segmented bones attached with wires and pins. The process controls to make the hand included proper drying of FibreTuff before 3D printing. VosFox Medical in the Netherlands is now printing feet with each bone segmented to show realistic construction. see website www.vosfoxmedical.com
FibreTuff Biocompatible compounds for 3D printing made with ISO 13485 Certification
FibreTuff has now produced it’s biocompatible compound with ISO 13485 certification. The company will be able to produce filaments for 3D Printing to use in surgical guides and implants. FibreTuff has global recognition to produce the most advanced 3D Printed anatomical bone like models for clinical and pre surgical assessment.
FibreTuff has global recognition to produce the most advanced 3D Printed anatomical bone like models for clinical and pre surgical assessment.
Filament printing with FibreTuff
3D printing in the Z direction is an absolute for bone like construction. The cortical bone will have 100% infill and cancellous bone being less with different infill patterns available through printer manufacturer.
FibreTuff Medical Grade filament processing for 3D Printing on Ultimaker 3 and S5
FibreTuff needs to be desiccant dried for 8-10 hrs. for optimum printing performance. It can be 3D Printed with various break away materials or support structure polymers to help with the vertical printing in Z direction. Standard breakaway for the Ultimaker 3D Printers can be used.
Recommended processing conditions.
The nozzle size should be .4 or .5mm with temps at 235 - 240C.
The printer bed should be heated at 80 -100C.
Cooling Fan on for first few layers than turned off
Recommended layer height .1 - .2mm
Printer oven is desired - temps 80C.
Printing speeds 45 -50 mm/sec
Density or infill is at 90 - 93% infill
Recommend Adhesive or breakaway - PVA
Please note: A new spool of FibreTuff Medical Grade filament is packaged in a vacuum sealed package with desiccant and will require some desiccant drying. If you leave spool exposed to elements over a period of time estimated at 3-4 days, moisture pickup is possible and may effect adhesion for layering. An indication of too much moisture is over extrusion or nodules present. It is recommended to desiccant dry spool for 10 hrs before printing.
Ultimaker Material Alliance Marketplace
FibreTuff medical grade filaments is a participant on the Ultimaker 3D Printers Materials Marketplace
Find out more
Ultimaker Transformation Summit - April 20, 2021
Bart van As of Ultimaker describes FibreTuff "bone like" filament for 3D printing. Robert Joyce nominated for 2021 Innovator.
Robert Joyce nominated for 2021 Innovator.
FibreTuff Applications and data
FibreTuff II testing for medical use
FibreTuff medical grade filament prints w no offensive odors
FibreTuff medical grade filament prints w no offensive odors
FibreTuff II has passed Cytotoxicity and Skin irritation tests and USP Class VI testing for temporary implants performed by NAMSA a regulatory approved testing
company for medical devices and implants.
FibreTuff medical grade filament prints w no offensive odors
FibreTuff medical grade filament prints w no offensive odors
FibreTuff medical grade filament prints w no offensive odors
FibreTuff medical grade filaments can be 3D printed with no offensive odors at recommended temperatures. Matter of fact there is no maleic in some filament that can cause skin irritation.
Matter of fact there is no maleic in some filament that can cause skin irritation.
Processing FibreTuff II on a FDM Printer
Multi axis printing of FibreTuff to achieve bone like qualities
Multi axis printing of FibreTuff to achieve bone like qualities
FibreTuff II medical grade filament requires a heated bed 80 -110 c . You can purchase FibreTuff II in two sizes, a 1.75 of 3.0mm. For the FibreTuff II to adhere to the heated bed a glue or adhesive sheet is required. The glue stick recommended is Cube Glue. GeckoTek is a supplier of adhesive sheet for heated beds to 3D Print. The nozzle size recommended for FibreTuff II are .5 with a processing temperature from 220 to 225C. If you use a .4 nozzle, higher heats may be necessary at 230C. A 3D Printer oven is preferred at temperatures of 80C for maximum efficiency.
If you use a .4 nozzle, higher heats may be necessary at 230C. A 3D Printer oven is preferred at temperatures of 80C for maximum efficiency.
Multi axis printing of FibreTuff to achieve bone like qualities
Multi axis printing of FibreTuff to achieve bone like qualities
Multi axis printing of FibreTuff to achieve bone like qualities
FibreTuff has been printed with various printers and software. Additional information to print FibreTuff with more than 3 axis of freedom is available.
FibreTuff processing
FibreTuff compounds for medical electronics
GeckoTek build plate works well with FibreTuff
GeckoTek build plate works well with FibreTuff
3D printed FibreTuff compounds for capacitive touch applications. See 3D Printer nScrypt Utilizes FibreTuff's PEEK Alternative PAPC 3D Printing Filaments
See 3D Printer nScrypt Utilizes FibreTuff's PEEK Alternative PAPC 3D Printing Filaments
GeckoTek build plate works well with FibreTuff
GeckoTek build plate works well with FibreTuff
GeckoTek build plate works well with FibreTuff
FibreTuff medical grade filament requires a heated build plate on a 3D Printer. Utilizing EZ-STIK GeckoTek build plate with 225C nozzle temperature and heated bed at 95C with PAPC II is preferred. https://www.geckotek.co/collections/build-plates/products/ez-stik-hot-build-plate
Glue to increase bed adhesion with FibreTuff filament
3D Printing FibreTuff filaments will require adhesive to heated bed
3D Printing FibreTuff filaments will require adhesive to heated bed
To adhere the FibreTuff medical grade filament to a 3D Printer heated bed - PVA water soluble breakaway, a glue stick or adhesive sheet is required. Magigoo is a preferred adhesive https://magigoo.comCube Glue is a good choice for increasing bed adhesion with FibreTuff medical grade filament.
Magigoo is a preferred adhesive https://magigoo.comCube Glue is a good choice for increasing bed adhesion with FibreTuff medical grade filament.
3D Printing FibreTuff filaments will require adhesive to heated bed
3D Printing FibreTuff filaments will require adhesive to heated bed
3D Printing FibreTuff filaments will require adhesive to heated bed
All FibreTuff biomaterials for FDM 3D Printed parts require a heated bed. The heated bed temperatures recommended for the FibreTuff I can range from 80 C to 110 C.
FibreTuff 3D Printer filament for medical applications
Mechanical FibreTuff I (pdf)
Download
3D Printed materials for anatomical bone models. (pdf)
(pdf)
Download
FibreTuff : CTScan 3D Life prints (pdf)
Download
Marshall University_LAMES-Bone : FibreTuff (pdf)
Download
Ohio State University : Finger Bone (pdf)
Download
Bioactiveglass:HaPeek: FibreTuff (pdf)
Download
ORS poster - Fibretuff_AT (jpg)
Download
FibreTuff I Medical Grade Filament
TDS Technical Data Sheet (pdf)
Download
SDS FibreTuff 1 Natural (pdf)
Download
FibreTuff II Medical Grade Filament
FibreTuff II Data Sheet (pdf)
Download
NAMSA : FibreTuff II Certificate (pdf)
Download
3D printing patient specific models using FibreTuff
Dr Hartman of OMFS used FibreTuff for 3D printing patient specific models in resident training and practice prior to larger, complex dental implant cases. The attached clip is a model surgery where he places zygomatic implants with the X-Guide dynamic navigation machine. He segmented the patient's CBCT and then printed it out of FibreTuff material. Dr Hartman is impressed with FibreTuff - It doesn't heat up and melt, produce burs like other filaments and its easy to drill and replicates real bone.
The attached clip is a model surgery where he places zygomatic implants with the X-Guide dynamic navigation machine. He segmented the patient's CBCT and then printed it out of FibreTuff material. Dr Hartman is impressed with FibreTuff - It doesn't heat up and melt, produce burs like other filaments and its easy to drill and replicates real bone.
FibreTuff Medical Grade Filament 3D Printed on UltimakeR S5
Ultimaker S5 Printer had utilized nylon parameters with FibreTuff medical grade filament for 3D Printing the part shown in the video at RAPID 2019 . The FibreTuff showed remarkable adhesion that utilized cellulose fiber in the biomaterial composition.
nScrypt 3D Print a skull cap with FibreTuff
nScrypt has 3D Printed a skull cap with bone like qualities using FibreTuff medical grade filament.
FibreTuff Medical Grade Filament 3D Printed on Air Wolf
The Air Wolf Axiom 3D printed FibreTuff medical grade filament at processing speed 45mm/sec to produce a calcaneus bone. The nozzle dia. .5mm at 235c temperature. The heated bed set at 100c. No oven chamber required.
Safety first - choose the right filament for your 3D printer
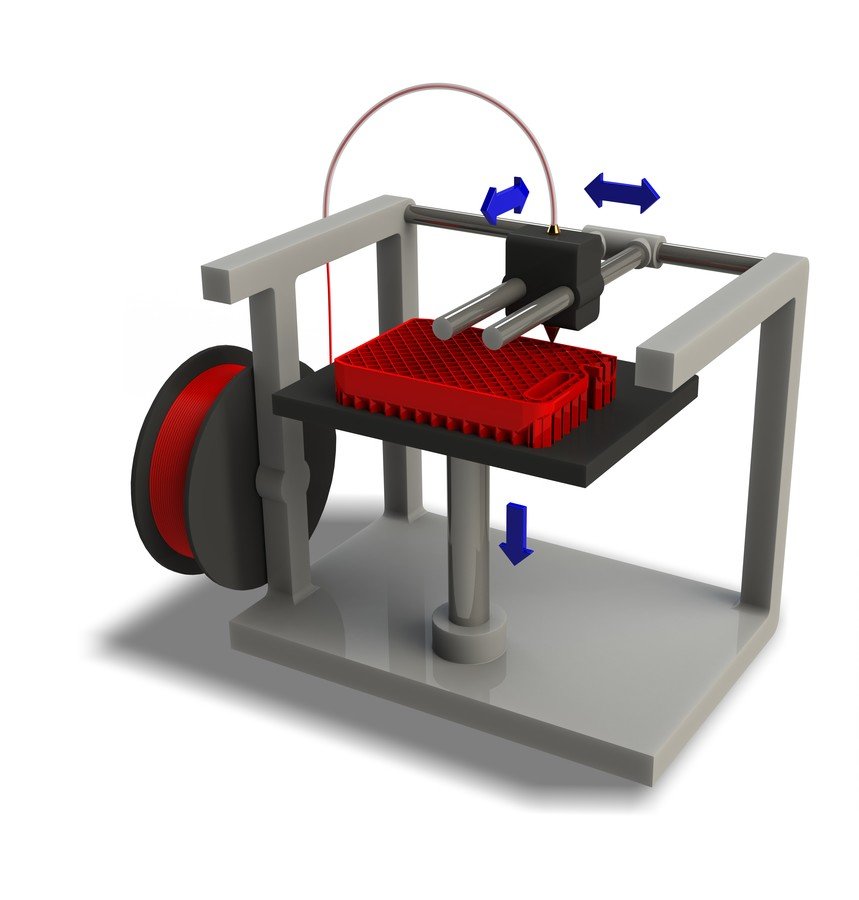
The sheer number of 3D printing applications is overwhelming. These devices have revolutionized the way we design and manufacture everything from replica body parts to firearms, robotics and food. And you - yes, you - can be part of this second industrial revolution.
We've looked at 4 affordable 3D printers under $100 on the market right now, each with its own advantages and disadvantages. Did you also know that different media with different levels of biocompatibility can be used with these printers?
But what is biocompatibility? Simply put, biocompatibility is how something works with the human body without any side effects. When we talk about biocompatibility in the context of 3D printing, we are really looking at two things. First, is the thread porous? If so, it can feed bacteria (and water), which is bad. Second, is it a chemical leak? If so, then it is clearly not biocompatible.
When we talk about biocompatibility in the context of 3D printing, we are really looking at two things. First, is the thread porous? If so, it can feed bacteria (and water), which is bad. Second, is it a chemical leak? If so, then it is clearly not biocompatible.
Some materials are more biocompatible than others, and there are other considerations to consider, including whether the material is shatter resistant. If you're planning on eating your 3D printed creation, or if you're designing a toy for a younger relative, you'll want to read this article.
PLA
PLA stands for polylactic acid and is a filament commonly used in 3D printing. It has two types of rigidity, one of which is much more resistant to fracture and flexible than the other.
The production of PLA is exciting, as quite often it is obtained from a by-product of corn, and not from oil processing. It is often used for medical purposes, including sutures and prostheses, due to its excellent biocompatibility and lack of harmful interaction with the human body. As a result, if you plan on making your own cutlery or toys, PLA is a great choice.
As a result, if you plan on making your own cutlery or toys, PLA is a great choice.
Nylon 618 and 645
Did you know that Nylon is a portmanteau of New York and London? It's right. It is also true that Nylon has made several appearances on the market, and since its invention in the 1930s, it has seen widespread use in both consumer and military applications.
Nylon is truly an amazing material. It has long been famous for its biocompatibility, especially in the medical field. Most cartilage replacements are made with this material, as well as quite a few dentures. While not as easy to find as PLA, nylon filaments are completely safe for human use. Body hackers rejoice!
Wood
Wood filament is not as common as ABS or PLA. It is made up of resin which is part of the wood pulp and part of PLA as a binding agent.
As mentioned earlier, PLA is biocompatible. Wood is also biocompatible, however it is a porous material that can absorb more water and bacteria than Billy Mays in the Zorbeez commercial. As a result, I would recommend that you exercise great care (if not restraint) when using it as a material for making cutlery or children's toys.
As a result, I would recommend that you exercise great care (if not restraint) when using it as a material for making cutlery or children's toys.
Unfair mentions
Two other popular filaments in use today are ABS (acrylonitrile butadiene styrene) and PC (polycarbonate). While both have excellent uses, both being fairly tough and readily available, neither is biocompatible.
While it is possible to get food grade polycarbonate, if you are 3D printing it is likely that you are using industrial grade material instead. This can be quite unpleasant, and it is known to release BPA at room temperature, which, according to 2003 studies, has adverse effects in mice.
Output
Choosing the right filament when 3D printing is essential. As always, safety is paramount and some fibers are safer than others. Did I miss something? Let me know in the comments below.
Photo credit: Oranse
3D printing plastic
Author: Igor Vilkhovsky. Publication date: . Category: News.
Publication date: . Category: News.
Hi-Tech workshop of our technopark is equipped with the most modern equipment, and, of course, it could not do without 3D printers. Most of the time our 3D printers are in operation. They print various figurines, machine parts and much, much more.
Children love to work with them, because in the process of printing you can see the real magic of modern technology. Right from scratch, layer by layer, they see how a product appears, which until recently was only a computer model.
Our students know how to draw 3D models, create a task from them - G-code - for a 3D printer, know how to start printing and know how to improve the adhesion of the printer desktop. And also, they know how to choose the right material for a particular print. This article will be about the most popular materials from which a 3D printer prints models.
A 3D printing consumable is called a filament. It is a plastic thread of various diameters. The thread can consist of various types of plastic. Now let's talk about the main types of plastics that are used in our technopark...
PLA (Polylactide) is a very environmentally friendly plastic. The raw materials for its production are resources such as corn and sugar cane. This plastic is biodegradable and biocompatible. It can be used to create tableware, biodegradable packaging and various containers. Also, it is possible to use it in medicine, for example, to create pins. It is one of the most popular materials for 3D printing. Does not shrink when printed.
ABS (acrylonitrile butadiene styrene) is an impact resistant plastic. It is a technical thermoplastic resin. Used when printing functional objects that will be used frequently. Due to the low cost of raw materials for production, ABS is one of the most affordable plastics.