3D printing titanium implants
Titanium: the 3D printing metal of the future in the medical sector?
3D printing news Medical Titanium: the 3D printing metal of the future in the medical sector?
Published on November 13, 2020 by Carlota V.
Sandvik’s additive manufacturing and metal powder specialists are preparing for the future of medical implants. The high-tech and global engineering group is currently unlocking the potential of 3D printed titanium devices for the medical industry at their cutting-edge titanium powder plants in Sandviken, Sweden. Additionally, during summer 2020, Sandvik’s specialist powder plant was awarded the ISO 13485:2016 medical certification for its Osprey titanium powders, positioning its highly automated production process at the forefront of medical device development.
If you follow developments in medicine, you will have noticed that medical implant technology has developed vastly over the years. Today, additive manufacturing is a technology with the potential to transform the way we treat patients. In fact, medical implant developers require a manufacturing technology that delivers speed, individualisation and the ability to produce complex designs. Therefore, 3D printing technologies, paired with biocompatible materials like titanium, are demonstrating their evident potential in creating life-changing solutions for patients.
Harald Kissel, R&D Manager at Sandvik Additive Manufacturing
Titanium 3D Printing
“Titanium, 3D printing and the medical sector are the perfect match. Titanium has excellent properties and is one of few metals accepted by the human body, while 3D printing can rapidly deliver bespoke results for an industry where acting quickly could be the difference between life and death,” explains Harald Kissel, R&D Manager at Sandvik Additive Manufacturing.
The latest advances in software also allow to leverage the benefits of 3D printing even more; Harald Kissel explains that by using computer tomography, it is possible to optimise designs that simply cannot be produced using other manufacturing methods. “What’s more, we can make our designs lighter, with less material waste and in shorter lead times,” he adds. Therefore, patients could receive a perfectly matching device, in less time and using a high-performing, lightweight material.
“What’s more, we can make our designs lighter, with less material waste and in shorter lead times,” he adds. Therefore, patients could receive a perfectly matching device, in less time and using a high-performing, lightweight material.
In fact, Sandvik believes that custom cranial implants and bespoke medical prosthesis are not for the distant future: the technology needed to develop and manufacture them already exists. Additionally, the medical certification they achieved will allow the company’s medical customers to complete the necessary regulatory supplier approvals when bringing a medical application to market. You can learn more about Sandvik’s projects in the medical sector HERE. The video below will also give you an idea of the impact of combining titanium and 3D printing in the medical field:
What do you think of these latest developments in the medical sector? Let us know in a comment below or on our Facebook and Twitter pages! Sign up for our free weekly Newsletter, all the latest news in 3D printing straight to your inbox!
New study assesses effectiveness of 3D printed titanium implants on bone growth
0Shares
A group of researchers from Korean hospitals have carried out a retrospective study to verify the effectiveness and safety of patient-specific 3D printed titanium implants on maxillofacial bones.
A total of 16 patients were observed during the study, each undergoing reconstructive surgery for various maxillofacial defects. The patients were fitted with customized titanium implants and received long term follow-ups over the course of numerous months.
Out of the 28 implants fitted, only one failed to unite successfully with the bone while the others showed “satisfactory” outcomes for the treatment of various oral and maxillofacial defects.
Titanium reconstruction of tumor-induced mandibular defects. Image via Nature.3D printing maxillofacial implants
3D printing has been used to produce guides that enhance patient outcomes for some time, and has more recently began to be leveraged on an experimental basis to create facial grafts that can be implanted into patients’ skulls. For instance, scientists at Texas A&M University have developed a novel 3D printed scaffold that directly facilitates bone cell growth after surgery.
Regarding more end-use applications, the Québec Industrial Research Centre (CRIQ) has deployed a GE Additive Arcam Q10 3D printer to expedite its production of patient-specific lower jawbone implants, while researchers at Paulista University, have 3D printed a facial prosthetic for a Brazilian cancer survivor, which included her entire right eye.
More recently, Health Canada approved its first Canadian-made 3D printed medical implant, a customizable mandibular plate for use in facial reconstruction surgery predominantly for patients with oral concer.
Dental implant on a titanium mandible. Image via Nature.The retrospective study
Autogenous bone grafting or implant placement is the primary method used to treat oral and maxillofacial defects, and while they are extensively biocompatible there can be issues regarding donor-site morbidity, surgical failure, and difficulty in reoperation.
Rapid advances in digital technology have opened up new avenues within the field or oral and maxillofacial surgery, with 3D printing enabling faster and accurate surgeries particularly regarding titanium materials that have already been verified for biocompatibility as dental implants.
The maxillofacial titanium implants used in the surgeries that the study recorded were 3D printed via electron beam melting (EBM) and selective laser sintering (SLS) processes. 28 implants were 3D printed in total and then inserted into the maxilla (dominant portion of the face), the mandible (lower jaw) or zygoma (cheek/temple) of 16 different patients.
28 implants were 3D printed in total and then inserted into the maxilla (dominant portion of the face), the mandible (lower jaw) or zygoma (cheek/temple) of 16 different patients.
The patients in question, of which seven were women and nine were men, ranged in age from nine years old to 78. A total of 28 defect areas were operated on, including five mandibular segments, nine zygomas, ten mandibular bodies, angles, or chins, and four maxillary areas.
A long-term follow-up process was then conducted, varying in length per patient from eight to 79 months. The study primarily analyzed the bone fusion of the titanium implant body, but also recorded postoperative infection, implant malunion, functional results, patient satisfaction, subsidence, osteolysis around the implants, and any complications that arose.
The study found that of the 28 implants, only one failed to unite with the bone. CBCT analysis showed that bone fusion at six months after surgery was 96.5 percent.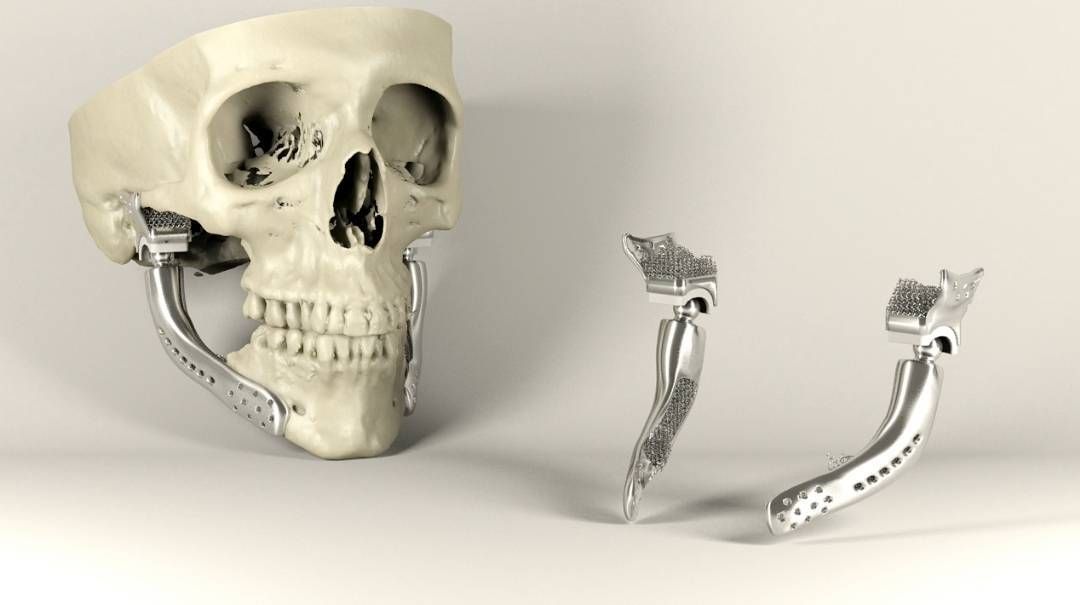 The study also observed no osteolysis – a progressive condition where bone tissue is destroyed – or subsidence around the titanium implants.
The study also observed no osteolysis – a progressive condition where bone tissue is destroyed – or subsidence around the titanium implants.
According to the study, the patients that took part in the trial were on the whole aesthetically and functionally satisfied with the results of their surgery. Although, two of the five patients who had a cheekbone reconstruction underwent revision surgery due to being dissatisfied with the appearance of the implant.
Photographs of patients with Treacher-Collins syndrome. Image via Nature.Reviewing the implants
The design of the bone-to-implant interface of the 3D printed implants was either a mesh or solid based on whether stability was required. As the rough surface of the mesh titanium implant was found to be more likely to integrate into the patient’s bone, this one was preferred.
SLS enables additive manufacturing with metals such as titanium at a high sintering temperature, however, owing to limited dimensional accuracy and poor surface roughness, process improvements are being made to improve its properties.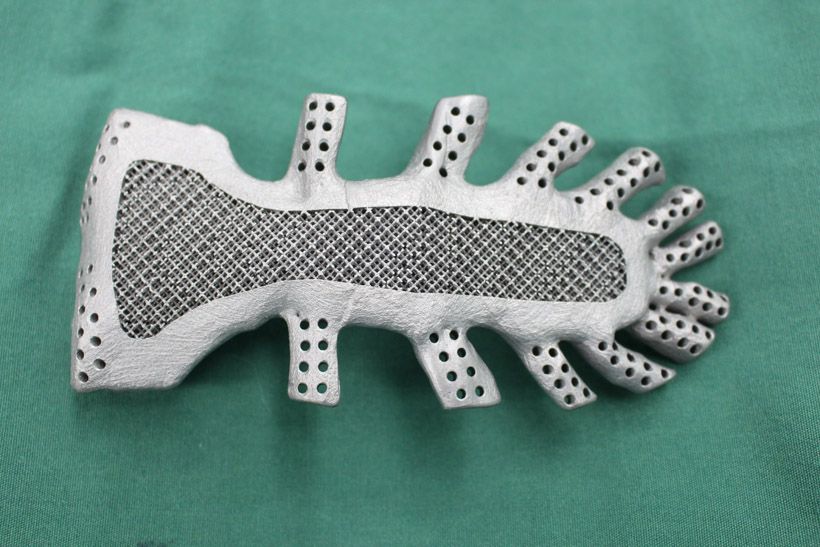 EBM uses an electron beam instead of a laser beam to sinter or to fuse the materials. EBM can be used to fabricate complex geometries by scanning each cross-sectional layer selectively, unlike SLS.
EBM uses an electron beam instead of a laser beam to sinter or to fuse the materials. EBM can be used to fabricate complex geometries by scanning each cross-sectional layer selectively, unlike SLS.
The implants 3D printed via EBM were more expensive than those 3D printed using SLS, however there was found to be no difference in the clinical results regarding the type of implant interface or the 3D printing method.
The follow-up appointments found that three out of the 16 patients experienced complications such as screw fractures and aesthetic dissatisfaction, however all of these were eventually resolved and post-operative recovery was found to be satisfactory in all patients.
Based on the patients’ experiences, the study has put forward guidelines for the use of 3D printed titanium patient-specific implants. In brief, the study advises that such implants should be used for continuity defects of the facial bone limited to hard tissue for which reconstruction has already been performed and there is no reconstruction option, and for cases where there is a mild or moderate bone defect due to previous excessive bone preparation in a patient with facial osteoplasty.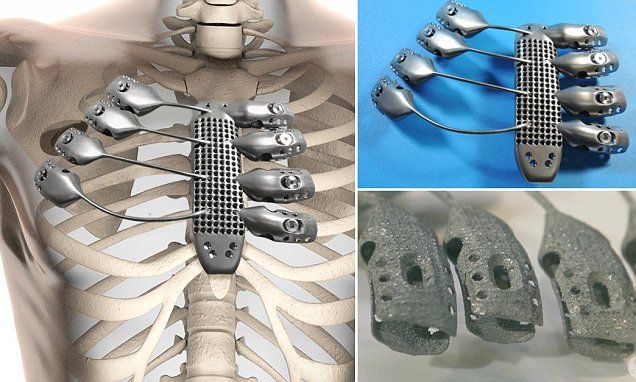
Additionally, the guidelines say that 3D printed titanium implants should also be used in cases of high aesthetic requirements such as correcting skeletal asymmetry, for areas that require functional load bearing such as the mandible, and when simultaneous reconstruction with dental implants is required.
Further information on the study can be found in the paper titled: “Reconstruction of maxillofacial bone defects using patient-specific long-lasting titanium implants,” published in the Nature journal. The study was co-authored by H. Lim, Y. Choi, W. Choi, I. Song, and U. Lee.
One of the restorative surgery case studies. Image via Nature.Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.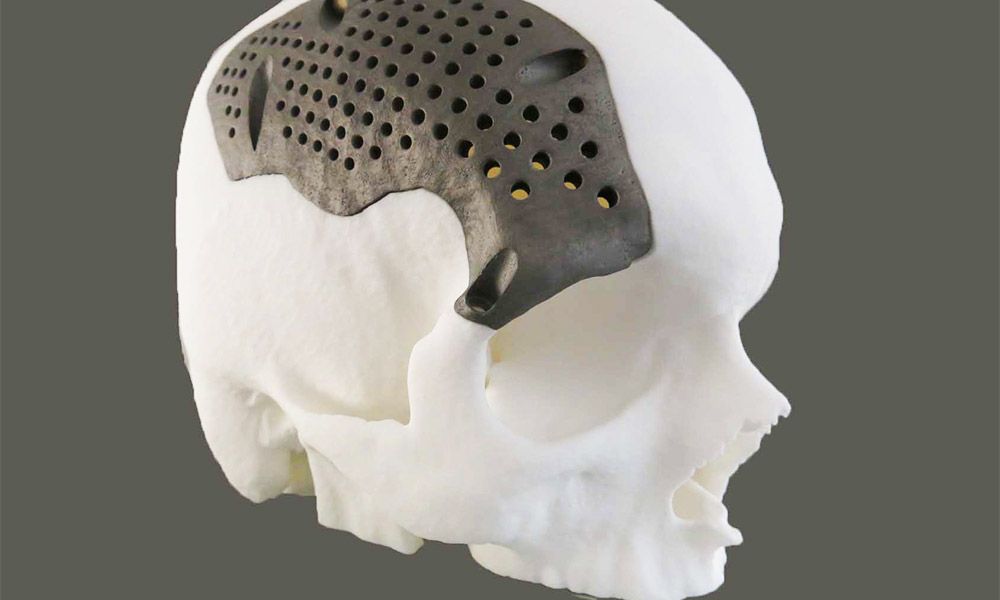
Subscribe to our YouTube channel for the latest 3D printing video shorts, reviews, and webinar replays.
Featured image shows titanium reconstruction of tumor-induced mandibular defects. Image via Nature.
Tags GE Additive H. Lim Health Canada I. Song Paulista University Quebec Industrial Research Centre Texas A&M University W. Choi Y. Choi
Hayley Everett
Hayley is a Technology Journalist for 3DPI and has a background in B2B publications spanning manufacturing, tools and cycling. Writing news and features, she holds a keen interest in emerging technologies which are impacting the world we live in.
Spinal implant fabrication using EP-M250 metal 3D printer
May 27, 2020
subscribe subscribe
As additive manufacturing technologies continue to evolve, so do their applications and availability. While rapid prototyping and tooling has always been at the forefront, the ultimate goal was to be able to apply 3D printing to industry. The medical world is no exception. After the first application of 3D printing in the field of medicine was prototypes and templates, technology has finally reached the ability to create customized implants using modern 3D printers. nine0011
The medical world is no exception. After the first application of 3D printing in the field of medicine was prototypes and templates, technology has finally reached the ability to create customized implants using modern 3D printers. nine0011
SHINING 3D has always remained true to its vision of making customized 3D solutions accessible to everyone. With the latest line of metal 3D printers, SHINING 3D has caught the attention of partners in the medical field who are now using this technology on an incredible scale. One of the pioneers who are paving the way in the creation and use of medical implants is Mantiz.
Mantiz is a manufacturer of medical devices using advanced technical solutions and technologies to ensure a pain-free and active life for all patients with diseases of the spine. The company has received KFDA (Medical Device Approval) certification and is listed on HIRA (Health Insurance Review and Assessment) in South Korea. In 2018, with government approval and funding, Mantiz began developing 3D printed cage implants. May 20191st, the company launched the Partner cage 3D printing system for posterior/transforaminal/oblique lateral/anterior lumbar interbody fusion surgery. The entire process of manufacturing the form of implants is carried out in Mantiz independently. The ability to avoid having to outsource the manufacturing process to a third party saves time, money and reduces the possibility of manufacturing errors.
In 2018, with government approval and funding, Mantiz began developing 3D printed cage implants. May 20191st, the company launched the Partner cage 3D printing system for posterior/transforaminal/oblique lateral/anterior lumbar interbody fusion surgery. The entire process of manufacturing the form of implants is carried out in Mantiz independently. The ability to avoid having to outsource the manufacturing process to a third party saves time, money and reduces the possibility of manufacturing errors.
Cage is a specially designed intervertebral disc replacement placed between the vertebrae to support the spine. Represents a structure for implantation in the form of a small hollow cylinder with many holes. Manufactured from titanium, ceramic, high performance PEEK polymer and filled with an osteoinductive material. It is used in surgery for stabilization and fusion of the vertebral bodies. In the treatment of diseases of the spinal column, a worn-out intervertebral disc is replaced with a cage. This operation is called "interbody fusion". nine0012
This operation is called "interbody fusion". nine0012
Mantiz uses SHINING 3D's EP-M250 Metal 3D Printer to produce titanium 3D printed cages for use in implant surgery. Cages are designed to specification: size, material, shape and porosity are all vital to implant performance. The finished cage design is loaded into the printer software where it is prepared for printing. Using the entire construction area of the EP-M250 3D printer, more than 50 individual implants can be produced in one printing operation. After implantation, the surrounding bone and muscle tissues begin to fuse with the implant, creating a strong structure in the patient's spine. nine0003
Industrial Metal 3D Printer EP-M250
“We have completed the development of a more advanced 3D printed titanium cage using the EP-M250 metal 3D printer. The results of mechanical tests confirm the safety and functionality of our implants.
The average closed porosity of a solid titanium 3D printed part is 3%. This leads to accelerated attachment of proteinaceous and mesenchymal stem cells for bone fusion,"
says Hongwon Yoon, inventor of the Partner 3D printing system and CTO of Mantiz.
The whole process from design to printing and from printing to implantation can be seen in the images below.
Design and 3D printing process: cage implant design in professional CAD software
Part preparation in 3D printing software
Use 3D printer for EP-M250 metal from ShINING 3D
Process Metal in 3D printer
created using 3D printing Cage
Only after a series of mechanical tests, the 3D printed cage can be used in surgery
After completing the complete design - 3D printing - post-processing - testing cycle, Partner can be used in a surgical operation.
 Partner is currently being used in the treatment of patients
Partner is currently being used in the treatment of patients Thanks to 3D printing capabilities, the Partner titanium cage (medium porosity) has been successfully used to optimize bone ingrowth in spinal surgery with an average pore size of 630–730 µm on the surface in contact with the end plate vertebral body, and the average range of porosity of the mesh parts is 70–80%. nine0003
Partner Mesh made on EP-M250 3D printer under microscope
The universe of 3D printing is rapidly expanding, and as technologies improve, so do their applications. The ability to manufacture custom parts in-house has never been so accessible. The automotive, aerospace, and medical industries are currently using 3D printing in manufacturing, and in this post, we've only scratched the surface of what additive technologies are capable of. Dipol specialists are ready to share additional information on how 3D printing can be applied in everyday work. nine0003
nine0003
Ural Federal University Electronic Scientific Archive: Additive 3D Printing Technologies in the Production of Titanium Implants and Plastic Compression Testing of Obtained Materials : master's thesis
Please use this identifier to cite or link to this item: http://hdl.handle.net/10995/63676
| Title: | Additive 3d printing technologies in the production of titanium implants and plastic compression testing of the obtained materials: master's thesis 9with. — Bibliography: p. 64-69 (52 titles). |
| Abstract: | The subject of the study is a titanium implant. The research method consists in the approximate solution of the boundary value problem by the finite element method in the ABAQUS software module. A patent and literature search in the field of application of additive technologies in the production of implants. The first section presents the results of the review.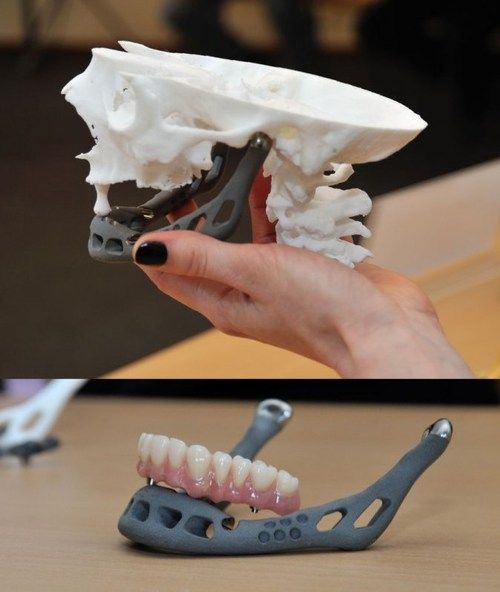 Were considered methods of additive manufacturing and methods of testing porous materials, the results are described in the second section. A description of the test for precipitation of a porous implant and a finite element simulation of the process of precipitation of cellular material have been carried out. As a result, the distribution scheme of the parameters of the stress-strain state was constructed, which allows estimating dangerous sections from the standpoint of structural failure. The obtained data can be applied in building the architecture of a set of unit cells with a description of the stress-strain state. nine0027 The subject of the study is a titanium implant. The research method consists in the approximate solution of the boundary value problem by the finite element method in the ABAQUS software module. A patent-literature search was carried out in the field of application of additive technologies in the production of implants. The first section presents the results of the survey. Were considered methods of additive manufacturing and methods of testing porous materials, the results are described in the second section. A description of the test for precipitation of a porous implant and a finite element simulation of the process of precipitation of cellular material have been carried out. As a result, the distribution scheme of the parameters of the stress-strain state was constructed, which allows estimating dangerous sections from the standpoint of structural failure. The obtained data can be applied in building the architecture of a set of unit cells with a description of the stress-strain state. nine0027 The subject of the study is a titanium implant. The research method consists in the approximate solution of the boundary value problem by the finite element method in the ABAQUS software module. A patent-literature search was carried out in the field of application of additive technologies in the production of implants. The first section presents the results of the survey. Additive manufacturing methods and methods for testing porous materials were considered, the results are described in the second section. A description of the upsetting test of a porous implant and finite element modeling of the process of upsetting a cellular material is carried out. As a result, a distribution scheme of the parameters of the stress-strain state was constructed, which makes it possible to evaluate dangerous sections from the standpoint of structural failure. The obtained data can be applied in building the architecture of a set of elementary cells with a description of the stress-strain state. nine0118 Additive manufacturing methods and methods for testing porous materials were considered, the results are described in the second section. A description of the upsetting test of a porous implant and finite element modeling of the process of upsetting a cellular material is carried out. As a result, a distribution scheme of the parameters of the stress-strain state was constructed, which makes it possible to evaluate dangerous sections from the standpoint of structural failure. The obtained data can be applied in building the architecture of a set of elementary cells with a description of the stress-strain state. nine0118 |
| Keywords: | MASTER'S THESIS IMPLANT CELLULAR STRUCTURE ADDITIVE TECHNOLOGIES TITANIUM ALLOYS 3D PRINTING POROSITY MODULUS OF ELASTICITY МАГИСТЕРСКАЯ ДИССЕРТАЦИЯ ИМПЛАНТАТ ЯЧЕИСТАЯ СТРУКТУРА АДДИТИВНЫЕ ТЕХНОЛОГИИ ТИТАНОВЫЕ СПЛАВЫ 3D ПЕЧАТЬ ПОРИСТОСТЬ MODULUS OF ELASTICITY |
| URI: | http://hdl. |