3D printing regulation
Medical 3D Printing: the main regulations
Defining Medical 3D Printing
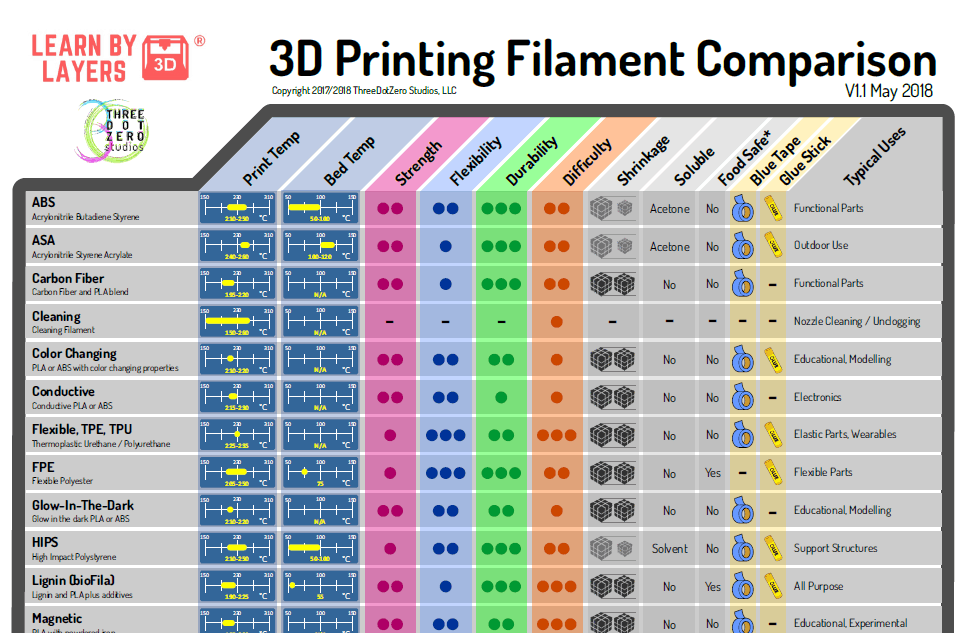
Simply put, 3D medical printing is the process of creating medical devices, transplants, surgical tools, and other medical products from a digital 3D model through additive manufacturing.
Medical 3D Printing Applications
There are numerous 3D printing applications in the field of medicine. Some of the more recent advances have proven that:
- 3D printing can generate faster and more affordable organ transplants
- 3D printing can speed up surgical procedures
- 3D printing can produce cheaper versions of high-quality surgical tools
Owing to the fast-paced developments in medical 3D printing technology, the regulatory space for these products is also (gradually) developing. Here, we will discuss the regulations that apply to 3D printing applications in medicines.
Medical 3D Printing Regulations in the US
The FDA focuses on regulating 3D-printed medical products based on product type, intended use, and potential risks to patients. As of today, 3D-printed medical products are regulated in the US market as follows:
- Medical devices are regulated by the FDA Center for Devices and Radiological Health (CDRH)
- Biologics are regulated by the FDA Center for Biologics Evaluation and Research
- Drugs are regulated by the FDA Center for Drug Evaluation and Research
Let us look at each of these categories, one by one:
3D-Printed Medical Devices
At present, medical devices are the most common type of product made using 3D printing.
The FDA expects all devices to adhere to current good manufacturing practices and ensure a finished device meets required quality specifications and an adequate level of quality. Therefore unless exempted, all device manufacturers must follow FDA guidance documents and Quality Systems regulations.
The FDA's Center for Devices and Radiological Health requires 3D-printed medical devices to be classified based on their risk level and the regulatory controls necessary to provide a reasonable assurance of safety and effectiveness. Accordingly, the products are classified into one of three regulatory categories as follows:
Accordingly, the products are classified into one of three regulatory categories as follows:
Class I devices are low risk
For example, products such as bandages and handheld surgical instruments fall into this Class. Most Class I devices are exempt from undergoing an FDA review before entering the market, known as premarket review, provided they comply with manufacturing and quality control standards.
Class II devices are considered moderate risk
Items such as infusion pumps fall in this Class. Some of the Class II devices have the advantage of being exempt from an FDA review. However, the majority of them undergo a 510(k) review (named for the relevant section of the Federal Food, Drug, and Cosmetic Act). In a 510(k) review, a manufacturer demonstrates 'substantial equivalence' of the product to an existing device on the market, thereby reducing the need for extensive clinical research.
Class III devices are considered moderate risk
Life-supporting or life-sustaining products that are substantially important in preventing impairment of human health or present an unreasonable risk of illness or injury fall in this Class.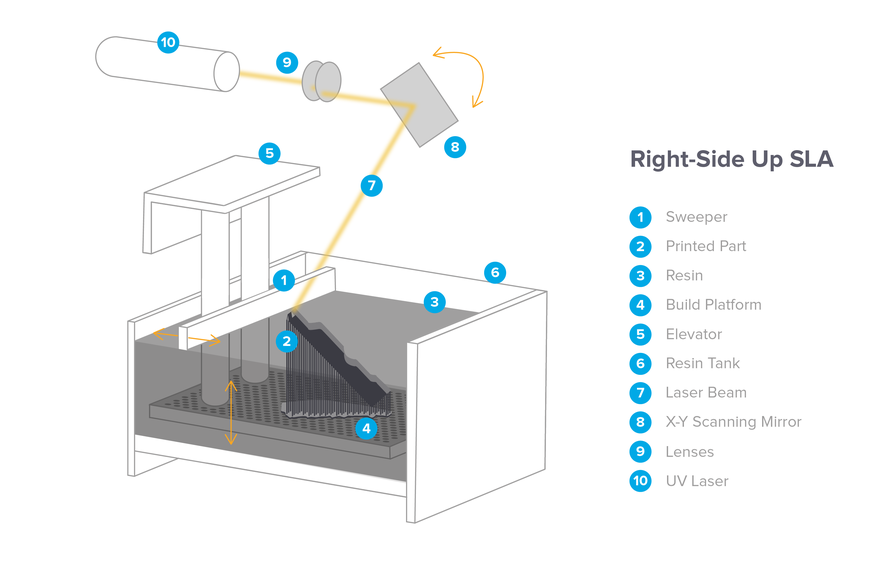 For example, a pacemaker is an example of a Class III device. Class III devices are subjected to higher regulatory scrutiny, and hence they must submit a complete application for premarket approval along with data from clinical trials. The FDA then evaluates whether sufficient scientific evidence exists to demonstrate that the new device is safe and effective for its intended use.
For example, a pacemaker is an example of a Class III device. Class III devices are subjected to higher regulatory scrutiny, and hence they must submit a complete application for premarket approval along with data from clinical trials. The FDA then evaluates whether sufficient scientific evidence exists to demonstrate that the new device is safe and effective for its intended use.
The higher the risk, the higher the Class, and the higher the regulatory scrutiny of a 3D-printed medical device.
In 2017, the FDA released guidance on:
-
-
- Application submissions for 3D-printed devices.
- Personalized devices such as joint replacements and cranial implants.
- Device and manufacturing process and testing considerations.
-
However, the guidance lacks information on point-of-care manufacturing, which may be a significant gap as the hospitals have rapidly invested in 3D printers over the past few years. Under the medical devices category, the FDA has also cleared software programs specifically intended to generate 3D models of a patient's anatomy; however, the FDA leaves it up to the medical facility to use that software correctly within the scope of its intended use.
Under the medical devices category, the FDA has also cleared software programs specifically intended to generate 3D models of a patient's anatomy; however, the FDA leaves it up to the medical facility to use that software correctly within the scope of its intended use.
3D-Printed Drugs
There is no specific FDA guidance for this category. At present, CDER’s Office of Pharmaceutical Quality is conducting research to understand the potential role of 3D printing in developing drugs. CDER has also been coordinating with pharmaceutical manufacturers to test this technology.
3D-Printed Biologics
CBER is actively interacting with stakeholders researching 3D printing for biological materials, such as human tissue, to understand potential challenges in the 3D printing of biological products. The FDA is also reviewing issues associated with bioprinting to evaluate the necessity of additional guidance outside the scope of the regulatory framework for regenerative medicine products.
Medical 3D Printing Regulations in the EU
3D printers are identified as a 'harmonized product' in the EU, requiring them to follow EU Product Harmonization Legislature. Here are some of the general legislatures that 3D medical printers may need to comply with, depending on the materials involved and the end-use case:
- Machinery Directive 2006/42/EEC addresses manufacturers, importers, and dealers of machinery regarding the safety of components and applies to new equipment. This directive aims at harmonizing the level of safety of products designed and manufactured by different manufacturers.
- Electromagnetic Compatibility Directive 2014/30/EC ensures that electrical and electronic equipment does not generate, or is not affected by, electromagnetic disturbance.
- Comprehensive EU legislation on chemicals is spearheaded by REACH and CLP, aiming to protect human health and the environment.
- WEEE 2012/19/EU is the directive on waste electrical and electronic equipment.

- RoHS II 2011/65/EU Directive is a directive on the restricted use of certain hazardous substances in electrical and electronic equipment.
- Directive (EU) 2017/2102 is an Amendment of Directive 2011/65/EU on the restricted use of certain hazardous substances in electrical and electronic equipment.
- REACH 1907/2006/EU seeks to improve the protection of human health and the environment through the better and earlier identification of the intrinsic properties of chemical substances.
Depending on the end-use of the product, the following EU legislations may apply to a specific medical 3D-printed product:
- Directive 93/42/EEC for medical devices
- Directive 98/79/EC for in-vitro diagnostics medical devices
- Directive 98/79/EC for active implant medical devices
Manufacturers of 3D-printed medical devices are required to carry out the necessary conformity assessment procedures, compose a technical file, draft the EU declaration of conformity, and affix the CE marking before entering the EU market.
The Road Ahead
3D printing is no longer some futuristic technology on the distant time horizon. Instead, the applications of 3D printing are rapidly increasing in the life sciences, which is driving the need for a regulatory framework.
Even though 3D printing regulations are still evolving, safety, efficacy, and quality remain the primary focus for approval by regulatory agencies. Companies in the pharmaceutical, biological, and medical device fields are familiar with this approach, and the current regulatory guidelines do provide some clarity on potential areas of concern. However, the guidelines need improvisations to accommodate manufacturing concerns at the point of care.
In the future, it may also be possible that the regulatory scope of 3D printing may not stop at the point of care but extend to patients, as 3D printing has the potential of empowering consumers to 3D print themselves. Therefore, it is clear that a lot of medical 3D printing regulations have already been put in place, but there is much more regulatory work ahead of us.
3D Printing of Medical Devices
Coronavirus COVID-19 Information Related to 3D Printing of Medical Devices
- FAQs on 3D Printing of Medical Devices, Accessories, Components, and Parts during the COVID-19 Pandemic
Overview
3D printing is a type of additive manufacturing. There are several types of additive manufacturing, but the terms 3D printing and additive manufacturing are often used interchangeably. Here we will refer to both as 3D printing for simplicity.
3D printing is a process that creates a three-dimensional object by building successive layers of raw material. Each new layer is attached to the previous one until the object is complete. Objects are produced from a digital 3D file, such as a computer-aided design (CAD) drawing or a Magnetic Resonance Image (MRI).
The flexibility of 3D printing allows designers to make changes easily without the need to set up additional equipment or tools. It also enables manufacturers to create devices matched to a patient’s anatomy (patient-specific devices) or devices with very complex internal structures. These capabilities have sparked huge interest in 3D printing of medical devices and other products, including food, household items, and automotive parts.
These capabilities have sparked huge interest in 3D printing of medical devices and other products, including food, household items, and automotive parts.
3D printed (left to right, top) models of a brain, blood vessel, surgical guide, and (bottom) medallion printed on FDA 3D printers.
Medical devices produced by 3D printing include orthopedic and cranial implants, surgical instruments, dental restorations such as crowns, and external prosthetics.
Due to its versatility, 3D printing has medical applications in:
- Medical devices regulated by FDA’s Center for Devices and Radiological Health (CDRH),
- Biologics regulated by FDA’s Center for Biologics Evaluation and Research, and
- Drugs regulated by FDA’s Center for Drug Evaluation and Research
Medical device manufacturers should refer to FDA guidance documents and Quality Systems regulations for more information on specific applications.
Additional Resources
- The 3Rs of 3D Printing: FDA's Role Learn how the FDA reviews and researches 3D printed medical products to protect the public health.

- Technical Considerations for Additive Manufactured Medical Devices - Guidance for Industry and Food and Drug Administration Staff (PDF - 803KB) FDA’s thinking on technical considerations specific to devices using additive manufacturing, the broad category of manufacturing encompassing 3D printing.
- How 3D Printers Work
A resource from the Department of Energy and includes descriptions of different types of printing processes - NIH 3D Print Exchange
Offers a unique set of models, learning resources and tutorials to create and share 3D-printable models related to biomedical science. The goal of the project is to facilitate the application of 3D printing in the biosciences. - American Society of the International Association for Testing and Materials (ASTM) International Committee F42 on Additive Manufacturing Technologies
This is a collaborative, consensus organization that has published standards and test methods for additive manufacturing and 3D printing.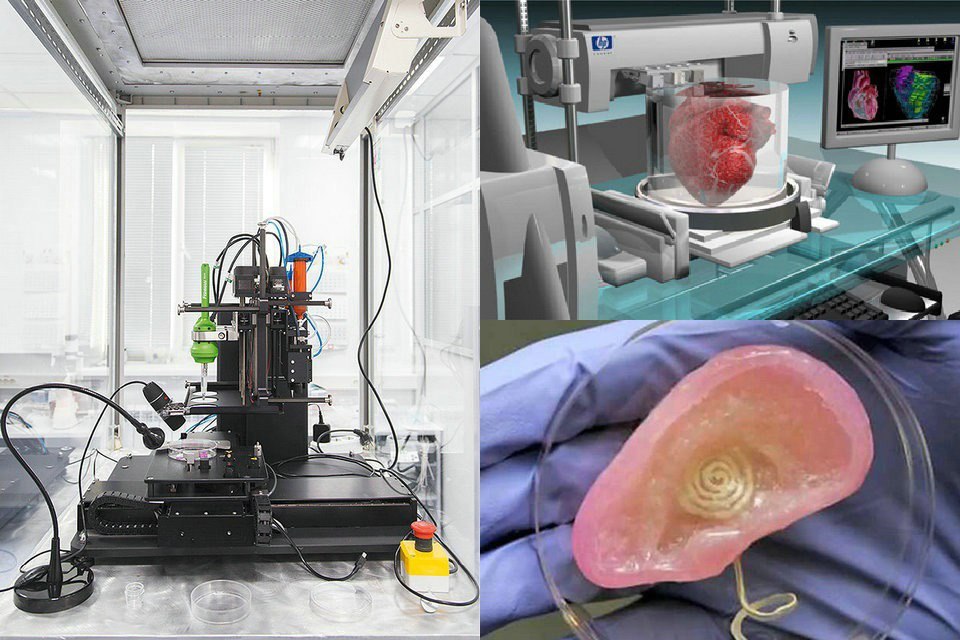
- America Make
A public private partnership whose members, including the FDA, are working together to innovate and accelerate 3D printing to increase our nation’s global manufacturing competitiveness.
The Masters Movement, IP Litigation and Law Reform
October 2019
Author: ), Brisbane, Australia
3D printing is an industry based on the principle of additive manufacturing (as opposed to the subtractive manufacturing principle that underlies the traditional manufacturing industry). 3D printing is also associated with the Craftsmen Movement, a social movement whose main idea is to develop designs for various products and share them.
Currently, the development of 3D printing is in a transitional phase. While the consumer “3D printing revolution” has failed, other varieties and categories of 3D printing have seen growth. (Photo courtesy of Queensland University of Technology)
(Photo courtesy of Queensland University of Technology) 3D printing is currently in transition. The consumer "3D printing revolution", which aimed to have a 3D printer in every home, has failed. MakerBot, a pioneer in 3D printing, is having trouble with its changing approach to intellectual property (IP) issues, disrupting its ties to the open source software community, and the user audience turned away from it. As former MakerBot CEO Bre Pettis said in an interview, "The open source community has kicked us out of their paradise." As a result, MakerBot was acquired by Stratasys, a leader in the 3D printing industry, which restructured and repurposed it.
Some other key players also went bankrupt. In particular, TechShop, a membership-funded and open-to-all network of studio-workshops for home craftsmen, went bankrupt. Maker Media, which publishes Make magazine and hosts craft festivals in the United States, has gone under external control. Make magazine founder Dale Doherty is trying to revitalize his project with a new structure he created called Make Community LLC.
Industrial 3D printing continues to grow
Although personal 3D printing has not developed as expected, there has been growth in a number of other forms and categories of 3D printing. Along with robotics and big data, 3D printing has become one of the promising technologies in the manufacturing industry. Companies specializing in information technology and design are working to improve the way 3D printing is used. Significant investments, especially from transport companies, have been attracted by the technology of 3D printing of metal products. In addition, there have been large-scale experiments related to the application of 3D printing in the healthcare sector, including 3D printing in dentistry, 3D printing in medicine, and bioprinting.
As technology improves and develops, there have been several cases of lawsuits being filed in the courts, as well as certain political developments regarding the regulation of the 3D printing industry. Our recently published book 3D Printing and Beyond explores some of the major developments in IC and 3D printing. In particular, it analyzes the issues of 3D printing in relation to areas such as copyright law, trademark law, patent law, and trade secrets (as well as some of the broader issues related to the regulation of 3D printing). In addition, the book highlights the use of open licensing mechanisms in the field of 3D printing.
In particular, it analyzes the issues of 3D printing in relation to areas such as copyright law, trademark law, patent law, and trade secrets (as well as some of the broader issues related to the regulation of 3D printing). In addition, the book highlights the use of open licensing mechanisms in the field of 3D printing.
3D printing and copyright law
A few years ago, there was a panic that the widespread use of 3D printing would lead to a wave of large-scale infringements of authors' rights, similar to the situation that arose with the advent of the Napster file-sharing network . Although such fears have not yet materialized, there have been various conflicts related to copyright and 3D printing. For example, Augustana College (United States) objected to 3D scanning of Michelangelo's statues, even though they were not subject to copyright protection and were clearly in the public domain. The American cable television network HBO has blocked the sale of an iPhone stand in the form of an "iron throne" from the TV series "Game of Thrones", made according to the drawings of designer Fernando Sosa using 3D printing. United States singer-songwriter Katy Perry has demanded a ban on the sale of a 3D-printed "shark on the left" figure by the same designer (nevertheless, this product subsequently reappeared in the Shapeways 3D Printing Systems catalog). The heirs of the French-American artist Marcel Duchamp opposed the production of a 3D-printed set of chess pieces based on the works of this artist.
United States singer-songwriter Katy Perry has demanded a ban on the sale of a 3D-printed "shark on the left" figure by the same designer (nevertheless, this product subsequently reappeared in the Shapeways 3D Printing Systems catalog). The heirs of the French-American artist Marcel Duchamp opposed the production of a 3D-printed set of chess pieces based on the works of this artist.
3D printing was also subject to the on-demand removal of content under the Digital Millennium Copyright Act (USA). Shapeways and a number of other 3D printing firms have raised concerns about the implications of this regime for online platforms and 3D printing intermediaries.
In addition, discussions took place on issues related to the use of technical protection measures in the context of copyright law and 3D printing. For example, the US Copyright Office has confirmed a limited technical protection exception for 3D printing stocks.
For example, the US Copyright Office has confirmed a limited technical protection exception for 3D printing stocks.
3D printing and design law
Developments in 3D printing have also raised the issue of product repair rights.
Efforts have been made across the European Union to recognize the right to repair in order to support consumer rights and develop a circular economy. In this regard, one of the important factors in achieving changes in the behavior of companies and consumers has become the European Greening Directive (Directive 2009/125/EC).
In July 2019, the United States Federal Trade Commission held a Hearing on "Can't be Repaired: A Workshop on Product Repair Restrictions." Significant differences remain between IP owners and right-to-repair advocates in the United States. Presidential candidate Elizabeth Warren has called for legislation to secure the right to repair for the benefit of farmers in the agricultural regions of the United States.
Significant and first-of-its-kind litigation in Australia regarding right to repair under Design Law ( GM Global Technology Operations LLC v S . . - S - Auto Parts Pty Ltd [2019] FCA 97). The Australian Treasury is considering policy options regarding the practice of sharing vehicle repair information.
Australian Capital Territory (ACT) Consumer Affairs Minister Shane Rettenbury called for recognition of the right to repair from the rostrum of the Consumer Affairs Forum, which includes ministers from both Australia and New Zealand. Federal Minister Michael Succar asked the Australian Productivity Commission to look into the matter.
Calls for right-to-repair laws, both at the provincial and federal levels, are also being heard in Canada. As Laura Tribe, Executive Director of Open Media, noted in this regard, “We are committed to ensuring that people have the opportunity to be the real owners of the products they own. ”
”
3D printing and trademark law
3D printing also brings uncertainty to trademark law and related legal regimes, including product substitution, identity rights, commercial use of characters, and trade dress. The legal conflict surrounding Katy Perry's trademark application for the "shark on the left" image provides some insight into some of the issues that arise in this regard.
Regarding bioprinting, Advanced Solutions Life Sciences sued Biobots Inc. Due to the alleged violation of its trademark rights ( Advanced Solutions Life Sciences , LLC V BIOBIOTS Inc 15 May 201111111111111. Advanced Solutions Life Sciences owns and uses the registered trademark Bioassemblybot for 3D bioprinting and tissue growth.
3D Printing and Patent Law
According to the 2015 WIPO Global Intellectual Property Report, Revolutionary Innovation and Economic Growth, 3D printing patent applications are on the rise. Some industrial 3D printing companies, including 3D Systems and Stratasys, have managed to build large 3D printing patent portfolios. Large industrial companies, including GE and Siemens, have also accumulated significant patent assets in 3D printing and additive manufacturing. Information technology companies, including Hewlett Packard and Autodesk, also play an important role in the 3D printing industry.
Some industrial 3D printing companies, including 3D Systems and Stratasys, have managed to build large 3D printing patent portfolios. Large industrial companies, including GE and Siemens, have also accumulated significant patent assets in 3D printing and additive manufacturing. Information technology companies, including Hewlett Packard and Autodesk, also play an important role in the 3D printing industry.
With the growing commercial importance of 3D printing in the manufacturing industry, there have been a significant number of litigations related to 3D printing of metal products. In July 2018, as part of the “Desktop Metal Inc.” v. Markforged, Inc. and Matiu Parangi (2018 Case No. 1:18-CV-10524), a federal jury found that Markforged Inc. did not infringe two patents owned by rival Desktop Metal Inc. (See Desktop Metal Inc. v. Markforged, Inc. and Matiu Parangi (2018) 2018 WL 4007724 (Massachusetts District Court, jury verdict). In this regard, the CEO of Markforged Inc. .” Greg Mark stated, “We are pleased with the jury's verdict that we have not infringed patents and that Metal X technology, which is the latest addition to the Markforged 3D printing platform, is based on our own Markforged's proprietary designs. " For its part, a spokesman for Desktop Metal noted that it was "satisfied that the jury recognized the validity of all claims in both Desktop Metal patents, which were discussed in a lawsuit against the company "Markforged"
" For its part, a spokesman for Desktop Metal noted that it was "satisfied that the jury recognized the validity of all claims in both Desktop Metal patents, which were discussed in a lawsuit against the company "Markforged"
In 2018 (after the above verdict) Desktop Metal Inc. and Markforged Inc. entered into a confidential financial agreement that settled all other litigation between them. However, in 2019 Markforged Inc. filed another lawsuit against Desktop Metal Inc. due to the fact that, according to her, the latter violated that part of the agreement, which concerned non-disclosure of negative information.
3D printing and trade secrets
In addition, the first litigation regarding 3D printing and trade secret legislation took place. In 2016, Florida-based 3D printing startup Magic Leap filed a lawsuit in federal court for the Northern District of California against two of its former employees for misappropriation of trade secret information within the meaning of Trade Secret Protection Act (“ Magic Leap Inc . " v Bradski et al (2017) case no. 5:16-cvb-02852). In early 2017, a judge granted the defendants' request to stay the case, stating that Magic Leap had failed to provide "a reasonable degree of specificity" to the disclosure of alleged trade secrets. Subsequently, the judge allowed Magic Leap to amend the text of its submission. In August 2017, the parties entered into a “confidential agreement” in connection with this issue. In 2019Mafic Leap sued the founder of Nreal for breach of contract, fraud, and unfair competition (Magic Leap Inc. v. Xu, 19-cv-03445, U.S. District Court for the Northern District of California (San Francisco)).
3D printing and open licensing
In addition to proprietary IP protections, 3D printing has a widespread practice of open licensing. Companies with a free distribution philosophy include Prusa Research (Czech Republic), Shapeways (Netherlands-US) and Ultimaker (Netherlands). Members of the Craft Movement used open licensing mechanisms to share and distribute 3D printing files. As noted in The State of the Commons 2017, the Thingiverse platform was one of the most popular platforms using Creative Commons licenses.
Other issues arising from the development of 3D printing
In addition to IP issues, the development of 3D printing also raises a number of other legal, ethical and regulatory issues. In healthcare, regulators have faced challenges with personalized medicine. The United States Food and Drug Administration and the Australian Health Products Administration held consultations on the development of a balanced set of regulations for medical 3D printing and bioprinting. The European Parliament has adopted a resolution calling for a comprehensive approach to the regulation of 3D printing.
Litigation regarding 3D printing of firearms is also ongoing in the United States. Several state attorneys general have sued the current administration to obstruct an agreement between the federal government and Defense Distributed. Several criminal cases have been filed in Australia, the United Kingdom, the United States and Japan in connection with attempts to 3D print firearms. Legislators are debating the feasibility of criminalizing crimes related to possession of digital blueprints for 3D printed firearms.
Footnotes
* Dr. Matthew Rimmer is Head of the KTU Research Program on Intellectual Property Law and Innovation and is involved in the KTU Research Center for Electronic Media, the KTU Australian Health Law Research Center and the KTU Research Program in International Law and global governance. In addition, he is a Senior Research Fellow at the Australian IP and 3D Printing Research Council Innovation Project. Dr. Rimmer is the author of numerous publications on copyright and information technology, patent law and biotechnology, access to medicines, anonymous packaging of tobacco products, IP and climate change, and IP of indigenous peoples. His current research interests include IP, Creative Industries and 3D Printing; IP and Public Health; and IP and Trade, including the Trans-Pacific Partnership, the Transatlantic Trade and Investment Partnership and the Agreement on Trade in Services. His work is held in the SSRN Abstracts and Bepress Selected Works archives and funded by the Australian Research Council.
Related Links
- Read more about 3D printing and IP Litigation
3D printing in healthcare: a regulatory perspective
However, unlocking the full potential of 3D printing for healthcare is challenging. Currently, the lack of a comprehensive regulatory framework for 3D printed medical and dental products is one of the biggest hurdles in the industry.
Several regulatory bodies are working to develop standards for 3D printing in healthcare. For example, the U.S. Food and Drug Administration (FDA) issued a guide in December 2017, “Technical Guidelines for Devices Made with Additives. ” The guide outlines technical considerations and recommendations for designing, manufacturing, and testing 3D printed medical devices.
Standardization of medical equipment - below we will consider the main issues.
There are three main classes of medical devices based on the level of harm they can cause to a patient.
Class I: Devices pose a low risk to the patient. Examples include nasal oxygen cannulas, hand-held stethoscopes, and hand splints.
Class II: These devices are more invasive and pose a moderate risk to the patient. Most medical devices are class II, including tracheal tubes, bone plates, and elbow prostheses.
Class III: These devices pose the greatest risk to the patient and include aortic valves, limited metal hip prostheses, and coronary stents.
While 3D printed prostheses may be classified as Class I, or low risk, technology has advanced, allowing the production of more advanced implants and instruments that embrace Class III, or high risk medical devices.
In order to certify Class I devices, manufacturers must prove that the final product is substantially identical to a device already on the market.
For Class II and III medical devices, the Food and Drug Administration and other regulatory agencies have not yet issued guidelines for premarket approval of 3D printed medical devices. These are innovative products and will likely require changes to the existing medical regulatory framework.
To date, the Food and Drug Administration has approved more than 100 medical devices, most of which are Class I medical devices.
Patient specific devices are the most difficult to regulate. Traditionally produced medical devices are standard, universal for all. However, when using a custom-made product, it can be difficult to test every single custom-made device.
Going forward, to create more opportunities for personalization, regulators should find ways to pre-approve user devices.
This is currently not easy as approval requirements are being developed for the certification of finished implants and instruments.