3D printing pharmacy
3D Printing in Pharmaceutical and Medical Applications – Recent Achievements and Challenges
1. ISO/ASTM 52900:2015(en) Additive manufacturing - General principles – Terminology.; 2018 March 26. Available from: https://www.iso.org/obp/ui/#iso:std:iso-astm:52900:ed-1:v1:en.
2. Gu D. Laser additive manufacturing of high-performance materials. Berlin: Springer; 2015. pp. 1–13. [Google Scholar]
3. Sachs EM, Haggerty JS, Cima MJ, Williams PA. Three dimensional printing techniques. In: US Patent US 5,204,055 A; 1993.
4. Jamroz W, Koterbicka J, Kurek M, Czech A, Jachowicz R. Application of 3D printing in pharmaceutical technology. Farm Pol. 2017;73(9):542–548. [Google Scholar]
5. Wu G, Wu W, Zheng Q, Li J, Zhou J, Hu Z. Experimental study of PLLA / INH slow release implant fabricated by three dimensional printing technique and drug release characteristics in vitro. Biomed Eng Online. 2014;13(97):1–11. [PMC free article] [PubMed] [Google Scholar]
6. Lee KJ, Kang A, Delfino JJ, West TG, Chetty D, Monkhouse DC, Yoo J. Evaluation of critical formulation factors in the development of a rapidly dispersing captopril oral dosage form. Drug Dev Ind Pharm. 2003;29(9):967–979. doi: 10.1081/DDC-120025454. [PubMed] [CrossRef] [Google Scholar]
7. Fina F, Madla CM, Goyanes A, Zhang J, Gaisford S, Basit AW. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int J Pharm. 2018;541(1–2):101–107. doi: 10.1016/j.ijpharm.2018.02.015. [PubMed] [CrossRef] [Google Scholar]
8. Wang J, Goyanes A, Gaisford S, Basit AW. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int J Pharm. 2016;503(1–2):207–212. doi: 10.1016/j.ijpharm.2016.03.016. [PubMed] [CrossRef] [Google Scholar]
9. Pere CPP, Economidou SN, Lall G, Ziraud C, Boateng JS, Alexander BD, et al. 3D printed microneedles for insulin skin delivery. Int J Pharm. 2018;544:425–32. [PubMed]
10. Clark EA, Alexander MR, Irvine DJ, Roberts CJ, Wallace MJ, Sharpe S, Yoo J, Haguea RJM, Tucka CJ, Wildman RD. 3D printing of tablets using inkjet with UV photoinitiation. Int J Pharm. 2017;529(1–2):523–530. doi: 10.1016/j.ijpharm.2017.06.085. [PubMed] [CrossRef] [Google Scholar]
3D printing of tablets using inkjet with UV photoinitiation. Int J Pharm. 2017;529(1–2):523–530. doi: 10.1016/j.ijpharm.2017.06.085. [PubMed] [CrossRef] [Google Scholar]
11. Kyobula M, Adedeji A, Alexander MR, Saleh E, Wildman R, Ashcroft I, Gellerte PR, Roberts CJ. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J Control Release. 2017;261(March):207–215. doi: 10.1016/j.jconrel.2017.06.025. [PubMed] [CrossRef] [Google Scholar]
12. Jamróz W, Kurek M, Łyszczarz E, Szafraniec J, Knapik-Kowalczuk J, Syrek K, Paluch M, Jachowicz R. 3D printed orodispersible films with aripiprazole. Int J Pharm. 2017;533(2):413–420. doi: 10.1016/j.ijpharm.2017.05.052. [PubMed] [CrossRef] [Google Scholar]
13. Arafat B, Wojsz M, Isreb A, Forbes RT, Isreb M, Ahmed W, Arafat T, Alhnan MA. Tablet fragmentation without a disintegrant: a novel design approach for accelerating disintegration and drug release from 3D printed cellulosic tablets. Eur J Pharm Sci. 10.1016/j.ejps.2018.03.019. [PubMed]
Eur J Pharm Sci. 10.1016/j.ejps.2018.03.019. [PubMed]
14. Li Q, Guan X, Cui M, Zhu Z, Chen K, Wen H, Jia D, Hou J, Xu W, Yang X, Pan W. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int J Pharm. 2018;535(1–2):325–332. doi: 10.1016/j.ijpharm.2017.10.037. [PubMed] [CrossRef] [Google Scholar]
15. Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. 3D printing of tablets containing multiple drugs with defined release profiles. Int J Pharm. 2015;494:643–50. [PubMed]
16. Huang W, Zheng Q, Sun W, Xu H, Yang X. Levofloxacin implants with predefined microstructure fabricated by three-dimensional printing technique. Int J Pharm. 2007;339(1–2):33–38. doi: 10.1016/j.ijpharm.2007.02.021. [PubMed] [CrossRef] [Google Scholar]
17. Yu DG, Branford-White C, Ma ZH, Zhu LM, Li XY, Yang XL. Novel drug delivery devices for providing linear release profiles fabricated by 3DP. Int J Pharm. 2009;370(1–2):160–166. doi: 10.1016/j.ijpharm. 2008.12.008. [PubMed] [CrossRef] [Google Scholar]
2008.12.008. [PubMed] [CrossRef] [Google Scholar]
18. Rowe CW, Katstra WE, Palazzolo RD, Giritlioglu B, Teung P, Cima MJ. Multimechanism oral dosage forms fabricated by three dimensional printing™ J Control Release. 2000;66(1):11–17. doi: 10.1016/S0168-3659(99)00224-2. [PubMed] [CrossRef] [Google Scholar]
19. Yu DG, Shen XX, Branford-White C, Zhu LM, White K, Yang XL. Novel oral fast-disintegrating drug delivery devices with predefined inner structure fabricated by three-dimensional printing. J Pharm Pharmacol. 2009;61(3):323–329. doi: 10.1211/jpp.61.03.0006. [PubMed] [CrossRef] [Google Scholar]
20. Fina F, Goyanes A, Gaisford S, Basit AW. Selective laser sintering (SLS) 3D printing of medicines. Int J Pharm. 2017;529(1–2):285–293. doi: 10.1016/j.ijpharm.2017.06.082. [PubMed] [CrossRef] [Google Scholar]
21. Martinez PR, Goyanes A, Basit AW, Gaisford S. Fabrication of drug-loaded hydrogels with stereolithographic 3D printing. Int J Pharm. 2017;532(1):313–317. doi: 10. 1016/j.ijpharm.2017.09.003. [PubMed] [CrossRef] [Google Scholar]
1016/j.ijpharm.2017.09.003. [PubMed] [CrossRef] [Google Scholar]
22. Muwaffak Z, Goyanes A, Clark V, Basit AW, Hilton ST, Gaisford S. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int J Pharm. 2017;527:161–170. doi: 10.1016/j.ijpharm.2017.04.077. [PubMed] [CrossRef] [Google Scholar]
23. Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J Control Release. 2015;217:308–314. doi: 10.1016/j.jconrel.2015.09.028. [PubMed] [CrossRef] [Google Scholar]
24. Khaled SA, Alexander MR, Wildman RD, Wallace MJ, Sharpe S, Yoo J, Roberts CJ. 3D extrusion printing of high drug loading immediate release paracetamol tablets. Int J Pharm. 2018;538(1–2):223–230. doi: 10.1016/j.ijpharm.2018.01.024. [PubMed] [CrossRef] [Google Scholar]
25. Goyanes A, Robles Martinez P, Buanz A, Basit AW, Gaisford S. Effect of geometry on drug release from 3D printed tablets. Int J Pharm. 2015;494(2):657–663. doi: 10.1016/j.ijpharm.2015.04.069. [PubMed] [CrossRef] [Google Scholar]
Int J Pharm. 2015;494(2):657–663. doi: 10.1016/j.ijpharm.2015.04.069. [PubMed] [CrossRef] [Google Scholar]
26. Genina N, Boetker JP, Colombo S, Harmankaya N, Rantanen J, Bohr A. Anti-tuberculosis drug combination for controlled oral delivery using 3D printed compartmental dosage forms: from drug product design to in vivo testing. J Control Release. 2017;268(August):40–48. doi: 10.1016/j.jconrel.2017.10.003. [PubMed] [CrossRef] [Google Scholar]
27. Maroni A, Melocchi A, Parietti F, Foppoli A, Zema L, Gazzaniga A. 3D printed multi-compartment capsular devices for two-pulse oral drug delivery. J Control Release. 2017;268(August):10–18. doi: 10.1016/j.jconrel.2017.10.008. [PubMed] [CrossRef] [Google Scholar]
28. Melocchi A, Parietti F, Loreti G, Maroni A, Gazzaniga A, Zema L. 3D printing by fused deposition modeling (FDM) of a swellable/ erodible capsular device for oral pulsatile release of drugs. J Drug Deliv Sci Technol. 2015;30:360–367. doi: 10.1016/j.jddst.2015.07.016. [CrossRef] [Google Scholar]
[CrossRef] [Google Scholar]
29. Sadia M, Sośnicka A, Arafat B, Isreb A, Ahmed W, Kelarakis A, Alhnan MA. Adaptation of pharmaceutical excipients to FDM 3D printing for the fabrication of patient-tailored immediate release tablets. Int J Pharm. 2016;513(1–2):659–668. doi: 10.1016/j.ijpharm.2016.09.050. [PubMed] [CrossRef] [Google Scholar]
30. Sadia M, Arafat B, Ahmed W, Forbes RT, Alhnan MA. Channelled tablets: an innovative approach to accelerating drug release from 3D printed tablets. J Control Release. 2018;269(November 2017):355–363. doi: 10.1016/j.jconrel.2017.11.022. [PubMed] [CrossRef] [Google Scholar]
31. Verstraete G, Samaro A, Grymonpré W, Vanhoorne V, Van Snick B, Boone MN, Hellemans T, Van Hoorebeke L, Remon JP, Vervaet C. 3D printing of high drug loaded dosage forms using thermoplastic polyurethanes. Int J Pharm. 2018;536(1):318–325. doi: 10.1016/j.ijpharm.2017.12.002. [PubMed] [CrossRef] [Google Scholar]
32. Kempin W, Franz C, Koster LC, Schneider F, Bogdahn M, Weitschies W, Seidlitz A. Assessment of different polymers and drug loads for fused deposition modeling of drug loaded implants. Eur J Pharm Biopharm. 2017;115:84–93. doi: 10.1016/j.ejpb.2017.02.014. [PubMed] [CrossRef] [Google Scholar]
Assessment of different polymers and drug loads for fused deposition modeling of drug loaded implants. Eur J Pharm Biopharm. 2017;115:84–93. doi: 10.1016/j.ejpb.2017.02.014. [PubMed] [CrossRef] [Google Scholar]
33. Li Q, Wen H, Jia D, Guan X, Pan H, Yang Y, Yu S, Zhu Z, Xiang R, Pan W. Preparation and investigation of controlled-release glipizide novel oral device with three-dimensional printing. Int J Pharm. 2017;525(1):5–11. doi: 10.1016/j.ijpharm.2017.03.066. [PubMed] [CrossRef] [Google Scholar]
34. Goyanes A, Wang J, Buanz A, Martínez-Pacheco R, Telford R, Gaisford S, et al. 3D printing of medicines: engineering novel oral devices with unique design and drug release characteristics. Mol Pharm. 2015;12(11):4077–4084. doi: 10.1021/acs.molpharmaceut.5b00510. [PubMed] [CrossRef] [Google Scholar]
35. Goyanes A, Det-Amornrat U, Wang J, Basit AW, Gaisford S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J Control Release. 2016;234:41–48. doi: 10.1016/j.jconrel.2016.05.034. [PubMed] [CrossRef] [Google Scholar]
J Control Release. 2016;234:41–48. doi: 10.1016/j.jconrel.2016.05.034. [PubMed] [CrossRef] [Google Scholar]
36. Scoutaris N, Ross SA, Douroumis D. 3D printed “starmix” drug loaded dosage forms for paediatric applications. Pharm Res. 2018;35(2):1–11. doi: 10.1007/s11095-017-2284-2. [PubMed] [CrossRef] [Google Scholar]
37. Markl D, Zeitler JA, Rasch C, Michaelsen MH, Müllertz A, Rantanen J, Rades T, Bøtker J. Analysis of 3D prints by X-ray computed microtomography and terahertz pulsed imaging. Pharm Res. 2017;34(5):1037–1052. doi: 10.1007/s11095-016-2083-1. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
38. Genina N, Holländer J, Jukarainen H, Mäkilä E, Salonen J, Sandler N. Ethylene vinyl acetate (EVA) as a new drug carrier for 3D printed medical drug delivery devices. Eur J Pharm Sci. 2016;90:53–63. doi: 10.1016/j.ejps.2015.11.005. [PubMed] [CrossRef] [Google Scholar]
39. Fu J, Yu X, Jin Y. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone.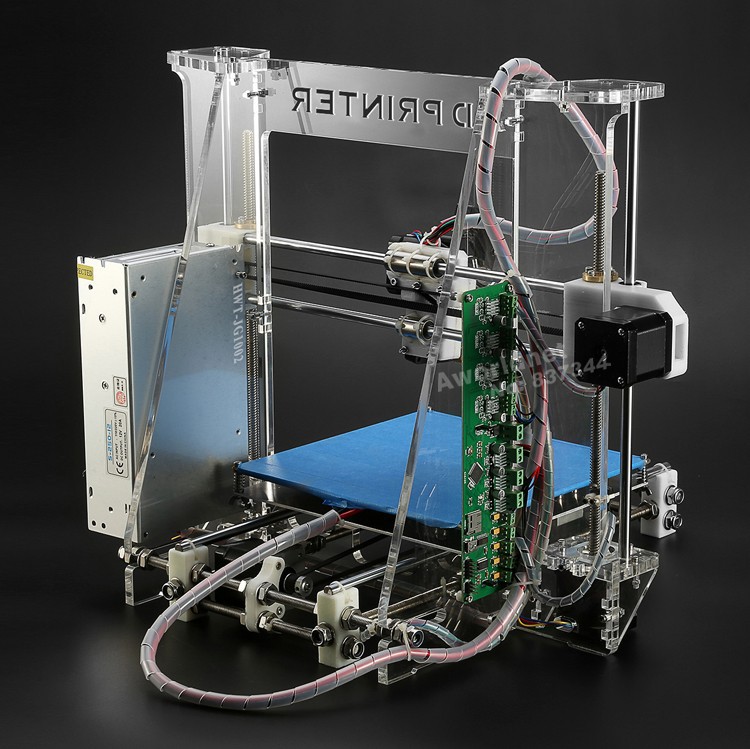 Int J Pharm. 2018;539(1–2):75–82. doi: 10.1016/j.ijpharm.2018.01.036. [PubMed] [CrossRef] [Google Scholar]
Int J Pharm. 2018;539(1–2):75–82. doi: 10.1016/j.ijpharm.2018.01.036. [PubMed] [CrossRef] [Google Scholar]
40. Holländer J, Genina N, Jukarainen H, Khajeheian M, Rosling A, Mäkilä E, et al. Three-dimensional printed PCL-based implantable prototypes of medical devices for controlled drug delivery. J Pharm Sci. 2016;105(9):2665–2676. doi: 10.1016/j.xphs.2015.12.012. [PubMed] [CrossRef] [Google Scholar]
41. Wu W, Zheng Q, Guo X, Huang W. The controlled-releasing drug implant based on the threedimensional printing technology: fabrication and properties of drug releasing in vivo. J Wuhan UnivTechnol-Mat Sci Edit. 2009;24(6):977–81.
42. Wu W, Zheng Q, Guo X, Sun J, Liu Y. A programmed release multi-drug implant fabricated by three-dimensional printing technology for bone tuberculosis therapy. Biomed Mater. 2009;4(6). Available from: stacks.iop.org/BMM/4/065005. [PubMed]
43. FDA. Application number: 207958Orig1s000 approval letter.; 2018 March 26. Available from: https://www. accessdata.fda.gov/drugsatfda_docs/nda/2015/207958Orig1s000Approv.pdf.
accessdata.fda.gov/drugsatfda_docs/nda/2015/207958Orig1s000Approv.pdf.
44. Brniak W, Jachowicz R, Krupa A, Skorka T, Niwinski K. Evaluation of co-processed excipients used for direct compression of orally disintegrating tablets (ODT) using novel disintegration apparatus. Pharm Dev Technol. 2013;8(2):464–474. doi: 10.3109/10837450.2012.710238. [PubMed] [CrossRef] [Google Scholar]
45. Aprecia Pharmaceuticals. Bringing ZipDose® Technology to life.; 2018 March 26. Available from: https://www.aprecia.com/zipdose-platform/3d-printing.php.
46. Goyanes A, Buanz ABM, Basit AW, Gaisford S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int J Pharm. 2014;476(1):88–92. doi: 10.1016/j.ijpharm.2014.09.044. [PubMed] [CrossRef] [Google Scholar]
47. Skowyra J, Pietrzak K, Alhnan MA. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur J Pharm Sci. 2015;68:11–17. doi: 10.1016/j.ejps.2014.11.009. [PubMed] [CrossRef] [Google Scholar]
48. Goyanes A, Chang H, Sedough D, Hatton GB, Wang J, Buanz A, Gaisford S, Basit AW. Fabrication of controlled-release budesonide tablets via desktop (FDM) 3D printing. Int J Pharm. 2015;496(2):414–420. doi: 10.1016/j.ijpharm.2015.10.039. [PubMed] [CrossRef] [Google Scholar]
Goyanes A, Chang H, Sedough D, Hatton GB, Wang J, Buanz A, Gaisford S, Basit AW. Fabrication of controlled-release budesonide tablets via desktop (FDM) 3D printing. Int J Pharm. 2015;496(2):414–420. doi: 10.1016/j.ijpharm.2015.10.039. [PubMed] [CrossRef] [Google Scholar]
49. Zhang J, Feng X, Patil H, Tiwari RV, Repka MA. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int J Pharm. 2017;519(1–2):186–197. doi: 10.1016/j.ijpharm.2016.12.049. [PubMed] [CrossRef] [Google Scholar]
50. Melocchi A, Parietti F, Maroni A, Foppoli A, Gazzaniga A, Zema L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int J Pharm. 2016;509(1–2):255–263. doi: 10.1016/j.ijpharm.2016.05.036. [PubMed] [CrossRef] [Google Scholar]
51. Okwuosa TC, Pereira BC, Arafat B, Cieszynska M, Isreb A, Alhnan MA. Fabricating a shell-core delayed release tablet using dual FDM 3D printing for patient-centred therapy. Pharm Res. 2017;34(2):427–437. doi: 10.1007/s11095-016-2073-3. [PubMed] [CrossRef] [Google Scholar]
Pharm Res. 2017;34(2):427–437. doi: 10.1007/s11095-016-2073-3. [PubMed] [CrossRef] [Google Scholar]
52. Goyanes A, Fina F, Martorana A, Sedough D, Gaisford S, Basit AW. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int J Pharm. 2017;527(1–2):21–30. doi: 10.1016/j.ijpharm.2017.05.021. [PubMed] [CrossRef] [Google Scholar]
53. Zema L, Melocchi A, Maroni A, Gazzaniga A. Three-dimensional printing of medicinal products and the challenge of personalized therapy. J Pharm Sci. 2017;106(7):1697–1705. doi: 10.1016/j.xphs.2017.03.021. [PubMed] [CrossRef] [Google Scholar]
54. Rams-Baron M, Jachowicz R, Boldyreva E, Zhou D, Jamroz W, Paluch M. Amorphous drugs benefits and challenges. Cham: Springer International Publishing AG; 2018. [Google Scholar]
55. Norman J, Madurawe RD, Moore CMV, Khan MA, Khairuzzaman A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv Drug Deliv Rev. 2017;108:39–50. doi: 10.1016/j.addr.2016.03.001. [PubMed] [CrossRef] [Google Scholar]
2017;108:39–50. doi: 10.1016/j.addr.2016.03.001. [PubMed] [CrossRef] [Google Scholar]
56. Boetker J, Water JJ, Aho J, Arnfast L, Bohr A, Rantanen J. Modifying release characteristics from 3D printed drug-eluting products. Eur J Pharm Sci. 2016;90:47–52. doi: 10.1016/j.ejps.2016.03.013. [PubMed] [CrossRef] [Google Scholar]
57. Sandler N, Preis M. Printed drug-delivery Systems for Improved Patient Treatment. Trends Pharmacol Sci. 2016;37(12):1070–1080. doi: 10.1016/j.tips.2016.10.002. [PubMed] [CrossRef] [Google Scholar]
58. Richey RH, Hughes C, Craig JV, Shah UU, Ford JL, Barker CE, Peak M, Nunn AJ, Turner MA. A systematic review of the use of dosage form manipulation to obtain required doses to inform use of manipulation in paediatric practice. Int J Pharm. 2017;518(1–2):155–166. doi: 10.1016/j.ijpharm.2016.12.032. [PubMed] [CrossRef] [Google Scholar]
59. Zajicek A, Fossler MJ, Barrett JS, Worthington JH, Ternik R, Charkoftaki G, et al. A report from the pediatric formulations task force: perspectives on the state of child-friendly oral dosage forms. AAPS J. 2013;15(4):1072–1081. doi: 10.1208/s12248-013-9511-5. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
AAPS J. 2013;15(4):1072–1081. doi: 10.1208/s12248-013-9511-5. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
60. Goyanes A, Scarpa M, Kamlow M, Gaisford S, Basit AW, Orlu M. Patient acceptability of 3D printed medicines. Int J Pharm. 2017;530(1–2):71–78. doi: 10.1016/j.ijpharm.2017.07.064. [PubMed] [CrossRef] [Google Scholar]
61. Park K. 3D printing of 5-drug polypill. J Control Release. 2015;217:352. doi: 10.1016/j.jconrel.2015.10.014. [PubMed] [CrossRef] [Google Scholar]
62. Yang Y, Wang H, Li H, Ou Z, Yang G. 3D printed tablets with internal scaffold structure using ethyl cellulose to achieve sustained ibuprofen release. Eur J Pharm Sci. 2018;115(December 2017):11–18. [PubMed] [Google Scholar]
63. Luzuriaga MA, Berry DR, Reagan JC, Smaldone, Ronald A., Gassensmith JJ. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab Chip 2018. 10.1039/C8LC00098K [PubMed]
64. McAllister DV, Wang PM, Davis SP, Park J-H, Canatella PJ, Allen MG, Prausnitz MR. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci. 2003;100(24):13755–13760. doi: 10.1073/pnas.2331316100. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci. 2003;100(24):13755–13760. doi: 10.1073/pnas.2331316100. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
65. Kaae S, Lind JLM, Genina N, Sporrong SK. Unintended consequences for patients of future personalized pharmacoprinting. Int J Clin Pharm. 2018;(123456789):1–4. [PubMed]
66. Qi S, Craig D. Recent developments in micro- and nanofabrication techniques for the preparation of amorphous pharmaceutical dosage forms. Adv Drug Deliv Rev. 2016;100:67–84. doi: 10.1016/j.addr.2016.01.003. [PubMed] [CrossRef] [Google Scholar]
67. Yoo J, Bradbury T, Bebb T, Iskra J, Surprenant H, West T. Three-dimensional printing system and equipment assembly. In: US Patent US 8,888,480 B2; 2014.
68. Trenfield SJ, Awad A, Goyanes A, Gaisford S, Basit AW. 3D printing pharmaceuticals: drug development to frontline care. Trends Pharmacol Sci. 2018:1–12. Available from:http://linkinghub.elsevier.com/retrieve/pii/S0165614718300440 [PubMed]
2018:1–12. Available from:http://linkinghub.elsevier.com/retrieve/pii/S0165614718300440 [PubMed]
69. FDA. Technical Considerations for Additive Manufactured Medical Devices - Guidance for Industry and Food and Drug Administration Staff. 2017. Available from: https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM499809.pdf.
70. Bracaglia LG, Messina MJ, Winston S, Kuo C-Y, Lerman M, Fisher JP. Printed pericardium hydrogels to promote wound healing in vascular applications. Biomacromolecules. 2017;18(11):3802–3811. doi: 10.1021/acs.biomac.7b01165. [PubMed] [CrossRef] [Google Scholar]
71. Herbert N, Simpson D, Spence WD, Ion W. A preliminary investigation into the development of 3-D printing of prosthetic sockets. J Rehabil Res Dev. 2005;42(2):141–146. doi: 10.1682/JRRD.2004.08.0134. [PubMed] [CrossRef] [Google Scholar]
72. Zuniga J, Katsavelis D, Peck J, Stollberg J, Petrykowski M, Carson A, Fernandez C. Cyborg beast: a low-cost 3d-printed prosthetic hand for children with upper-limb differences. BMC Res Notes. 2015;8:10. doi: 10.1186/s13104-015-0971-9. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
BMC Res Notes. 2015;8:10. doi: 10.1186/s13104-015-0971-9. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
73. del Rosario C. Casts made By 3-D printing make broken bones stylish. Medical Daily; 2013.
74. Lee JS, Hong JM, Jung JW, Shim JH, Oh JH, Cho DW. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication. 2014;6(2):024103. doi: 10.1088/1758-5082/6/2/024103. [PubMed] [CrossRef] [Google Scholar]
75. He Y, Xue G, Fu J. Fabrication of low cost soft tissue prostheses with the desktop 3D printer. Sci Rep. 2014;4:6973. doi: 10.1038/srep06973. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
76. Unkovskiy A, Spintzyk S, Brom J, Huettig F, Keutel C. Direct 3D printing of silicone facial prostheses: A preliminary experience in digital workflow. J Prosthet Dent. 2018; 10.1016/j.prosdent.2017.11.007. [PubMed]
77. del Junco M, Okhunov Z, Yoon R, Khanipour R, Juncal S, Abedi G, Lusch A, Landman J. Development and initial porcine and cadaver experience with three-dimensional printing of endoscopic and laparoscopic equipment. J Endourol 2015;29(1):58–62. [PMC free article] [PubMed]
J Endourol 2015;29(1):58–62. [PMC free article] [PubMed]
78. Zopf DA, Hollister SJ, Nelson ME, Ohye RG, Green GE. Bioresorbable airway splint created with a three-dimensional printer. N Engl J Med. 2013;368(21):2043–2045. doi: 10.1056/NEJMc1206319. [PubMed] [CrossRef] [Google Scholar]
79. Ma L, Zhou Y, Zhu Y, Lin Z, Chen L, Zhang Y, Xia H, Mao C. 3D printed personalized titanium plates improve clinical outcome in microwave ablation of bone tumors around the knee. Sci Rep. 2017;7:7626. doi: 10.1038/s41598-017-07243-3. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
80. Dzian A, Živčák J, Penciak R, Hudák R. Implantation of a 3D-printed titanium sternum in a patient with a sternal tumor. World J Surg Oncol. 2018;16:7. doi: 10.1186/s12957-018-1315-8. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
81. O'Brien EK, Wayne DB, Barsness KA, McGaghie WC, Barsuk JH. Use of 3D printing for medical education models in transplantation medicine: a critical review. Curr Transplant Rep. 2016;3(1):109–119. doi: 10.1007/s40472-016-0088-7. [CrossRef] [Google Scholar]
Curr Transplant Rep. 2016;3(1):109–119. doi: 10.1007/s40472-016-0088-7. [CrossRef] [Google Scholar]
82. Zein NN, Hanouneh IA, Bishop PD, Samaan M, Eghtesad B, Quintini C, Miller C, Yerian L, Klatte R. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19(12):1304–1310. doi: 10.1002/lt.23729. [PubMed] [CrossRef] [Google Scholar]
83. Witkowski JS, Pędziwiatr M, Major P, Budzyński A. Cost-effective, personalized, 3D-printed liver model for preoperative planning before laparoscopic liver hemihepatectomy for colorectal cancer metastases. Int J Comput Assist Radiol Surg. 2017;12(12):2047–2054. doi: 10.1007/s11548-017-1527-3. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
84. Silberstein JL, Maddox MM, Dorsey P, Feibus A, Thomas R, Lee BR. Physical models of renal malignancies using standard cross-sectional imaging and 3-dimensional printers: a pilot study. Urology. 2014;84(2):268–273. doi: 10.1016/j. urology.2014.03.042. [PubMed] [CrossRef] [Google Scholar]
urology.2014.03.042. [PubMed] [CrossRef] [Google Scholar]
85. Cheung CL, Looi T, Lendvay TS, Drake JM, Farhat WA. Use of 3-dimensional printing technology and silicone modeling in surgical simulation: development and face validation in pediatric laparoscopic Pyeloplasty. J Surg Educ. 2014;71(5):762–767. doi: 10.1016/j.jsurg.2014.03.001. [PubMed] [CrossRef] [Google Scholar]
86. Lokr T, Krieger A, Sable C, Olivieri L. Novel uses for three-dimensional printing in congenital heart disease. Curr Pediatr Rep. 2016;4(2):28–34. doi: 10.1007/s40124-016-0099-y. [CrossRef] [Google Scholar]
87. Sodian R, Schmauss D, Markert M, et al. Three-dimensional printing creates models for surgical planning of aortic valve replacement after previous coronary bypass grafting. Ann Thorac Surg. 2008;85:2105–2108. doi: 10.1016/j.athoracsur.2007.12.033. [PubMed] [CrossRef] [Google Scholar]
88. Valverde I. Three-dimensional printed cardiac models: applications in the field of medical education, cardiovascular surgery, and structural heart interventions. Rev Esp Cardiol. 2017;70(4):282–291. doi: 10.1016/j.recesp.2016.09.043. [PubMed] [CrossRef] [Google Scholar]
Rev Esp Cardiol. 2017;70(4):282–291. doi: 10.1016/j.recesp.2016.09.043. [PubMed] [CrossRef] [Google Scholar]
89. Marks M, Alexander A, Matsumoto J, Matsumoto J, Morris J, Petersen R, Jack J, Jr, Oishi T, Jones D. Creating three dimensional models of Alzheimer’s disease. 3D Print Med. 2017;3(13):1–11. [PMC free article] [PubMed] [Google Scholar]
90. Murphy SV, Atala A. 3D printing of tissues and organs. Nat Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [PubMed] [CrossRef] [Google Scholar]
91. Markstedt K, Mantas A, Tournier I, Martínez Ávila H, Hägg D, Gatenholm P. 3D bioprinting humanchondrocytes with nanocellulose? Alginate bioink for cartilage tissue engineering applications. Biomacromolecules. 2015;16:1489–96. [PubMed]
92. Skardal A, Devarasetty M, Kang H-W, Mead I, Bishop C, Shupe T, Lee SJ, Jackson J, Yoo J, Soker S, Atala A. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater. 2015;25:24–34. doi: 10.1016/j.actbio.2015.07.030. [PubMed] [CrossRef] [Google Scholar]
2015;25:24–34. doi: 10.1016/j.actbio.2015.07.030. [PubMed] [CrossRef] [Google Scholar]
93. Melhem MR, Park J, Knapp L, Reinkensmeyer L, Cvetkovic C, Flewellyn J, Lee MK, Jensen TW, Bashir R, Kong H, Schook LB. 3D printed stem-cell-laden, microchanneled hydrogel patch for the enhanced release of cell-secreting factors and treatment of myocardial infarctions. ACS Biomater Sci Eng. 2017;3:1980–1987. doi: 10.1021/acsbiomaterials.6b00176. [CrossRef] [Google Scholar]
94. Lee H, Cho D-W. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab Chip. 2016;16:2618–2625. doi: 10.1039/C6LC00450D. [PubMed] [CrossRef] [Google Scholar]
95. Johnson BJ, Lancaster KZ, Hogue IB, Meng F, Kong YL, Enquistc LW, McAlpine MC. 3D printed nervous system on a chip. Lab Chip. 2016;16:1393–1400. doi: 10.1039/C5LC01270H. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
96. Cui H, Nowicki M, Fisher JP, Zhang LG. 3D bioprinting for organ regeneration. Adv Healthc Mater. 2017;6:1601118. doi: 10.1002/adhm.201601118. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Adv Healthc Mater. 2017;6:1601118. doi: 10.1002/adhm.201601118. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
97. Khoo ZX, Teoh JEM, Liu Y, Chua CK, Yang S, An J, et al. 3D printing of smart materials: a review on recent progresses in 4D printing. Virtual Phys Prototyp. 2015;10(3):103–22.
98. Campbell TA, Tibbits B, Garrett B. The next wave: 4D printing programming the material world. Washington, DC: The Atlantic Council; 2014. p. 1–15.
99. Raviv D, Zhao W, McKnelly C, Papadopoulou A, Kadambi A, Shi B, Hirsch S, Dikovsky D, Zyracki M, Olguin C, Raskar R, Tibbits S. Active printed materials for complex self-evolving deformations. Sci Rep. 2014;4:7422. doi: 10.1038/srep07422. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
100. Gladman AS, Matsumoto EA, Nuzzo RG, Mahadevan L, Lewis JA. Biomimetic 4D printing. Nat Mat. 2016;15:413–418. doi: 10.1038/nmat4544. [PubMed] [CrossRef] [Google Scholar]
101. Han D, Lu Z, Chester SA, Lee H. Micro 3D printing of a temperature-responsive hydrogel using projection micro-stereolithography. Sci Rep. 2018;8:1963. doi: 10.1038/s41598-018-20385-2. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Sci Rep. 2018;8:1963. doi: 10.1038/s41598-018-20385-2. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
102. Ge Q, Sakhaei AH, Lee H, Dunn CK, Fang NX, Dunn ML. Multimaterial 4D printing with tailorable shape memory polymers. Sci Rep. 2016;6:31110. doi: 10.1038/srep31110. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
103. Gupta MK, Meng F, Johnson BN, Kong YL, Tian L, Yeh Y-W, Masters N, Singamaneni S, McAlpine MC. 3D printed programmable release capsules. Nano Lett. 2015;15:5321–5329. doi: 10.1021/acs.nanolett.5b01688. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
104. Feinberg AW. Biological soft robotics. Annu Rev Biomed Eng. 2015;17:243–265. doi: 10.1146/annurev-bioeng-071114-040632. [PubMed] [CrossRef] [Google Scholar]
105. Nawroth JC, Lee H, Feinberg AW, Ripplinger CM, McCain ML, Grosberg A, Dabiri JO, Parker KK. A tissue-engineered jellyfish with biomimetic propulsion. Nat Biotechnol. 2012;30:792–797. doi: 10.1038/nbt.2269. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
106. Phillips R, Purohit PK, Kondev J. Mechanics of biological nanotechnology. In: Bhushan B, editor. Springer handbook of nanotechnology. 2. Berlin: Springer; 2006. pp. 1199–1203. [Google Scholar]
Phillips R, Purohit PK, Kondev J. Mechanics of biological nanotechnology. In: Bhushan B, editor. Springer handbook of nanotechnology. 2. Berlin: Springer; 2006. pp. 1199–1203. [Google Scholar]
107. Alford PW, Feinberg AW, Sheehy SP, Parker KK. Biohybrid thin films for measuring contractility in engineered cardiovascular muscle. Biomaterials. 2010;31:3613–3621. doi: 10.1016/j.biomaterials.2010.01.079. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
108. Williams BJ, Anand SV, Rajagopalan J, Saif MTA. A self-propelled biohybrid swimmer at low Reynolds number. Nat Commun. 2014;5:1–8. [PubMed] [Google Scholar]
109. Tanaka Y, Sato K, Shimizu T, Yamato M, Okano T, Kitamori T. A micro-spherical heart pump powered by cultured cardiomyocytes. Lab Chip. 2007;7(2):207–212. doi: 10.1039/B612082B. [PubMed] [CrossRef] [Google Scholar]
110. Nagarajan N, Dupret-Bories A, Karabulut E, Zorlutuna P, Vrana NI. Enabling personalized implant and controllable biosystem development through 3D printing. Biotechnol Adv. 2018;36(2):521–533. doi: 10.1016/j.biotechadv.2018.02.004. [PubMed] [CrossRef] [Google Scholar]
Biotechnol Adv. 2018;36(2):521–533. doi: 10.1016/j.biotechadv.2018.02.004. [PubMed] [CrossRef] [Google Scholar]
111. Gioumouxouzis CI, Katsamenis OL, Bouropoulos N, Fatouros DG. 3D printed oral solid dosage forms containing hydrochlorothiazide for controlled drug delivery. J Drug Delivery Sci Technol. 2017;40:164–171. doi: 10.1016/j.jddst.2017.06.008. [CrossRef] [Google Scholar]
Merck, Aprecia, and FabRx on transitioning 3D printed pharmaceuticals from lab to clinic
Nominations for the 2021 3D Printing Industry Awards are now open, have your say who is leading the industry now.
“3D printing will be a basic tool in the virtuous cycle of personalized medicine.”
That’s the view of Alvaro Goyanes, Co-Founder and Director of pharmaceutical 3D printing specialist FabRx, whose recent co-authored paper evaluates both the state-of-play of 3D printed pharmaceuticals and the clinical potential of the technology.
The paper states that 3D printing offers several significant advantages for clinical pharmaceutical drug development, not least the ability to personalize medicines to individual patient needs, expedite drug delivery timelines, and provide on-demand medication to those in hospitals, pharmacies and hard-to-reach areas.
However, while these benefits have been widely demonstrated in recent years, the clinical potential of the technology is yet to be realized.
“Despite this growing support for printing technologies, regulatory and technical challenges still remain before the widespread adoption of this technology into the pharmaceutical industry will occur,” the paper states.
3D Printing Industry spoke to paper co-author, Goyanes and Anna Worsley from FabRx about the findings of the study and garnered insights from Christoph Huels, Founder Additive Manufacturing of Tablets at the Innovation Center of global pharmaceutical company Merck, and Head of Excipient Solid Application at Merck’s Life Science Business Sector, Finn Bauer, on how 3D printing pharmaceuticals can transition from the lab to real-world clinical applications in the coming years.
Additionally, Kirk Donaldson, Vice President Business Development & Alliance Management at Aprecia, the company behind the first FDA-approved 3D printed drug Spritam, shares why the company believes “it’s important for people to begin seeing 3D printed pharmaceuticals as modern technology” that is available today, as opposed to future technology.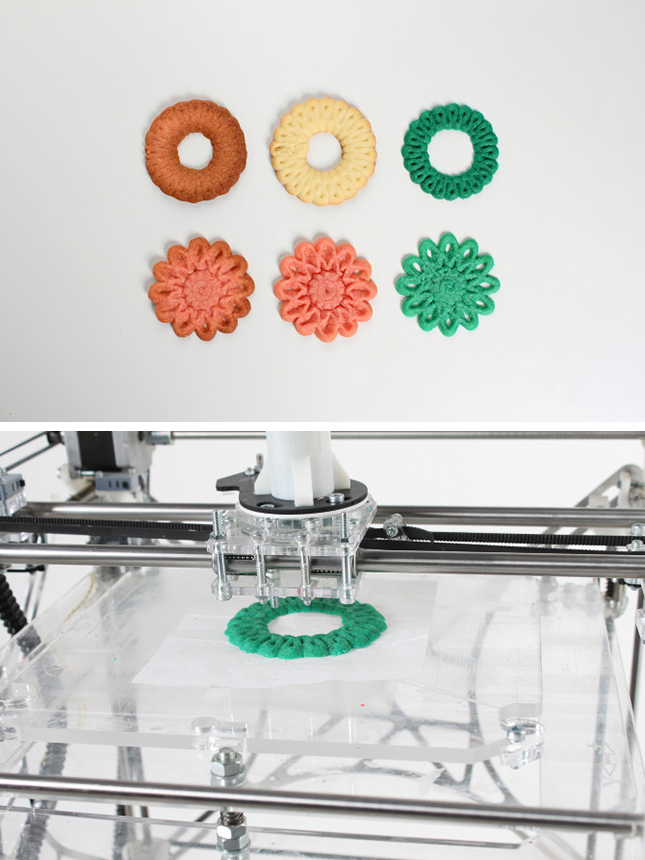
3D printing pharmaceuticals
3D printing can revolutionize the production of pharmaceuticals that target the gastrointestinal tract by offering a flexible drug manufacturing platform that can be adapted in response to changing markets and patient needs. Additive manufacturing makes possible the on-demand printing of personalized medicines on the front line in surgeries, hospitals, and hard-to-reach areas, while addressing issues of dosage inflexibility, high time and labor requirements, and large costs associated with conventional large-scale manufacturing processes, like tableting and encapsulation.
“Pharmaceutical 3D printing has many applications, we can say that it brings the manufacturing process closer to the patient, benefiting many different patient groups,” explains Goyanes. “It allows for the personalized dosing of drugs, removing the need to self-prepare medication or take multiple pills to each the prescribed, non-commercialized dose, an issue that arises for many treatment pathways that can lead to adverse effects. Pharmaceutical 3D printing also allows for personalized polypills, pills with multiple drugs combinations. This would benefit complex disorders and comorbidities.”
Pharmaceutical 3D printing also allows for personalized polypills, pills with multiple drugs combinations. This would benefit complex disorders and comorbidities.”
However, despite the widely demonstrated benefits of 3D printing pharmaceuticals, the clinical potential of the technology is yet to be realized, according to FabRx’s recent study. The paper highlights the main 3D printing technologies that are currently being leveraged for producing pharmaceuticals – binder jetting, vat polymerization, powder bed fusion (PBF), material jetting, and material extrusion.
“Each of the 3D printing technologies have different pros and cons, each being better suited to different specific applications, drugs and patient groups,” continues Goyanes. “Extrusion-based technologies like fused deposition modeling (FDM), semisolid extrusion or direct powder extrusion are probably more suited for printing at the dispensing point in hospitals and pharmacies. Other technologies included in vat photopolymerization like digital light processing (DLP) and stereolithography (SLA), could be more suitable for printing drug-loaded medical devices. ”
”
Binder jetting, for instance, forms the basis of Aprecia’s ZipDose additive manufacturing technology that produces its Spritam tablets. Binder jetting is particularly suited to producing highly porous, fast-dissolving tablets with high drug-loading profiles such as Spritam, but can also be applied for the production of complex formulations such as near zero-order release dosage forms. Vat polymerization technologies such as SLA, DLP and continuous liquid interface production (CLIP) are often chosen for the fabrication of drug delivery devices with complex structures, such as microneedles for skin delivery, bladder devices for intravesical drug delivery, hearing aids, and drug-loaded scaffolds, among other applications.
Elsewhere, PBF has been mostly restricted to the production of medical devices such as scaffolds due to the risk of drug degradation from the high localized temperatures required to sinter powder materials. However, last year FabRx successfully used an SLS 3D printing technique to fabricate orodispersible tablets designed with Braille and Moon patterns to aid medicine taking for patients with visual impairment.
Material jetting techniques have been deployed for the production of oral films, nano and microparticles with arbitrary geometries, and controlled-release tablets, while material extrusion technologies such as FDM have been used to manufacture hollow structures and tablets with different shapes. FDM also enables the modification of drug release patterns to vary the infill percentage and the controlled and immediate release dosage forms of 3D printed tablets.
“In the future it would be ideal to have several technologies available for clinical applications, preferably combined into one printer,” Goyanes adds. “This would allow for fully personalizable medications for more treatment pathways.”
FabRx has already developed what it claims is the first GMP (good manufacturing practice) ready pharmaceutical 3D printer, the M3DIMAKER, which is currently available for research purposes. Before the machine’s launch in 2020, the firm had already completed trials with the printer in hospitals, pharmacies, and research institutions around the globe.
“The M3DIMAKER is already taking part in national and international collaborations which will involve future clinical trials, and we are in communication with the MHRA to aid regulation development for this state-of-the-art technology,” Goyanes says. “It combines multiple 3D printing technologies into one machine, allowing for flexible use and increased personalization potential.”
Finn Bauer, Head of Excipient Solid Application at Merck’s Life Science Business Sector. Photo via Merck.3D printed pharmaceuticals in industry
In addition to the surge in research interest surrounding 3D printed pharmaceuticals in recent years, there have been a number of notable developments from companies looking to commercialize the technology, too. One such firm is Merck, which in 2020 embarked upon a joint project with EOS Group company AMCM to develop and produce 3D printed tablets, first for clinical trials and then later for commercial manufacturing.
“There are several initiatives ongoing related to 3D printing of oral solid dosage forms,” says Bauer. “Different 3D printing technologies, such as powder jetting, material extrusion, and laser sintering are explored at Merck. In addition, different excipients are tested and developed for their use in extrusion-based applications. Parteck MXP is a polymer for hot-melt extrusion in our Life Science Sector and its use in 3D printing has been described in various publications.”
“Different 3D printing technologies, such as powder jetting, material extrusion, and laser sintering are explored at Merck. In addition, different excipients are tested and developed for their use in extrusion-based applications. Parteck MXP is a polymer for hot-melt extrusion in our Life Science Sector and its use in 3D printing has been described in various publications.”
Founded way back in 1668, Merck is a significant Big Pharma player. The company’s growing interest in leveraging 3D printing for medicine development is therefore notable and could help to pave the way for greater commercialization of the technology in the future.
“Laser sintering is explored for the production of oral dosage forms in the Merck Innovation Center,” adds Huels. “We aim to make services available for the formulation of manufacturing of clinical trial supply in a first step. The team is planning to offer the technology to customers for exploratory studies in the course of the next year. In parallel, developments are on the way to build a 3D printer, able to work in a GMP environment. The GMP solution is expected to be ready for market introduction in two-to-three years.”
The GMP solution is expected to be ready for market introduction in two-to-three years.”
However, Huels points out that certain challenges still remain to scale up the 3D printing of medicines for commercial manufacturing, and fully translate the technology from the lab to clinics on the ground.
“Commercial manufacturing requires for so-called ‘blockbuster drugs’ and billions of tablets per year for global supply,” he says. “3D printing technologies are not able to secure the supply with the current throughput today. Significant improvements need to be done to reach that in the longer-term future. Nevertheless, the current throughput is already, or will be soon, well suited to supply orphan or smaller oncology indications, where only millions of tablets are required per year.”
In fact, Huels observes clinical supply as an application that can already be met by some 3D printing technologies, since only thousands of tablets are needed per clinical trial phase.
“Several developments are on the way to apply 3D printing for the drug development process,” he continues. “Therefore, it will only take a few years until more than the current drug (Spritam) will be supplied to patients via 3D printing. Smaller indications will be first, in which the use of 3D printing will have the most commercial viability.
“Therefore, it will only take a few years until more than the current drug (Spritam) will be supplied to patients via 3D printing. Smaller indications will be first, in which the use of 3D printing will have the most commercial viability.
Christoph Huels, Founder Additive Manufacturing of Tablets at the Innovation Center of Merck. Photo via Merck.“With further improvements in throughput and the decentralization of pharma production, a more generalized use of 3D printing for production may be observed.”
Achieving commercialization of 3D printed drugs
At the head of the field, the commercial potential of 3D printed pharmaceuticals is already being realized by Aprecia, with the company having received FDA approval for its 3D printed epilepsy medication Spritam back in 2015. The company has continued to innovate in this area since then, and in January entered into a partnership with non-profit R&D firm Battelle to increase its manufacturing output and advance its 3D printing system from clinical supply to commercial scale.
“Aprecia is actively engaged in exciting 3D printing research and development in several key areas including 3D printing formulation platforms for advanced, patient-centric dosage forms and the engineering of proprietary 3D printing systems based on improved processes and novel equipment trains,” says Donaldson. “Our first formulation platform (ZipDose) and enabling open-bed printing system is not only clinic ready, but also commercial ready.”
The company’s second formulation platform, ZipCup, reportedly expands Aprecia’s ability to handle a diverse set of materials while minimizing formulation time and risk. The platform, which Donaldson says will be clinic-ready within the next year and commercial-ready shortly afterward, can be delivered through either the original open bed or new in-cavity printing systems with some added process steps and equipment modifications. Regarding products, Donaldson says Aprecia has “several pipeline candidates” that will advance to clinic this year and next.
Since receiving FDA approval for Spritam, the company has closely observed how the 3D printed drug has been received by medical professionals, caregivers and patients.
“Neurologists have accepted Spritam as an alternative dosage form of levetiracetam for epilepsy patients that have difficulty swallowing tablets or do not like to take the liquid form,” Donaldson says. “Patients and their caregivers appreciate the rapid disintegration with a small sip of liquid and seem to remain both satisfied and persistent on the medication due to its ease of use. This has been the case across all age ranges.”
Regarding children, Donaldson says Spritam is an excellent way to bridge the gap from messy, difficult to measure, and sometimes unpleasant tasting liquid that they tend to start on, to a solid oral form as they get older.
“When introducing alternative dosage forms into a largely genericized market, both commercial and government payers may seek limits or restrictions to use so it’s imperative to make sure the overall value proposition resonates, and both physician and patient is supported and assisted with respect to coverage and reimbursement throughout the process,” he adds.
According to Donaldson, the real value of 3D printing is its ability to do things beyond the capabilities of conventional manufacturing technologies, such as overcoming formulation challenges and delivering personalized medicine closer to the site of care. However, he believes that further awareness of the technology’s potential for such applications still needs to be generated.
“There is always a need for greater awareness of the benefits to be gained with 3D printing technologies,” Donaldson says. “We need to expand awareness across the pharmaceutical industry at all levels of the organization through education and promotion. We need to generate more trial usage through feasibility studies to demonstrate our readiness. We need to gain market acceptance of novel dosage forms and personalized formulations through preference studies, market research projects, key opinion leader development, collaborations with other enabling pharmaceutical technologies, and ultimately successful in market products. ”
”
Making the move from lab to clinic?
According to FabRx’s study, the integration of 3D printing alongside other novel technologies into pharmaceuticals is forecast to give rise to a “new digital pharmacy era”. The technology has the potential to provide a digital and decentralized platform for the production of tailored medicines that could be delivered at the point of care. However, while new research papers evidencing the potential of 3D printing technologies in this area are being published almost daily, only a comparatively small number of studies have been performed in pre-clinical and clinical settings.
Regulatory, quality, and technical concerns, as well as some resistance to digital technologies in the Pharma sector, are likely to be the reasons for this, as to date Spritam remains the only 3D printed drug product approved for commercialization by the FDA. From a Big Pharma perspective, one concern surrounding digitalized 3D printing technologies for decentralized production may be the protection of formulation and process details, as well as issues surrounding data security and accessibility.
With well-established manufacturing processes developed more than two centuries ago, the pharmaceutical industry is also facing a mindset and culture shift in order to meet the growing demand for personalized oral medicines. The study suggests that while there is still some work to be done before all stakeholders have confidence in 3D printing for drug development, inviting major stakeholders, including clinicians, patients and Big Pharma, to come together in a multidisciplinary approach to increasing the adoption of 3D printing in the pharmaceutical sector could further pave the way forward for the technology into human and animal studies.
“In the next 10 years, we predict that pharmaceutical 3D printing for personalized dosing and drug combinations will become standard practice for a handful of more troublesome treatment pathways around the world,” Goyanes concludes. “As more and more medication and drug combinations are tested, pharmaceutical 3D printing will become more widespread and will enter local pharmacies for more common treatment pathways.
“3D printing will be a basic tool in the virtuous cycle of personalized medicine. It will simplify and personalize many treatments, improving lives and saving healthcare organizations millions of pounds every year.”
Further information on FabRx’s study into the state of play of pharmaceutical 3D printing can be found in the paper titled “Translating 3D printed pharmaceuticals: From hype to real-world clinical applications,” published in the Advanced Durg Delivery Reviews journal. The study is co-authored by I. Seoane-Viaño, S. Trenfield, A. Basit, and A. Goyanes.
The virtuous cycle of personalized medicine. Image via FabRx.Nominations for the 2021 3D Printing Industry Awards are now open, have your say who is leading the industry now.
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Subscribe to our YouTube channel for the latest 3D printing video shorts, reviews and webinar replays.
Featured image shows Aprecia 3D printed pills. Photo via Aprecia Pharmaceuticals.
3D drug printing
September 11, 2018
Popular science competition "Bio/mol/text"-2018
Overview
Tablet formed by printing layers of polymer mixed with drug
Alvaro Goyanes
-
Author
- Alexey Korolev
-
Editor
- Andrey Panov
Topics
- "Bio/mol/text"-2018
- Biology
- Biotechnology
- The medicine
- Personalized medicine
- Pharmacology
Bio/mol/text contest entry: Recently, 3D printing has become one of the most revolutionary and powerful tools in many areas. The pharmaceutical industry is no exception. This article will tell readers about the history of 3D printing in the pharmaceutical industry, the latest developments and achievements in this field, and the prospects for the development of 3D printing in the industry.
The pharmaceutical industry is no exception. This article will tell readers about the history of 3D printing in the pharmaceutical industry, the latest developments and achievements in this field, and the prospects for the development of 3D printing in the industry.
This work was published in the nomination "Biopharmaceuticals" of the competition "bio/mol/text"-2018.
The general sponsor of the competition is the Diaem company: the largest supplier of equipment, reagents and consumables for biological research and production.
The partner of the nomination is the medical company Invitro.
The audience award was sponsored by the Genotek Medical Genetics Center.
"Book" sponsor of the competition - "Alpina non-fiction"
Introduction
3D printers can now create just about anything. From car parts and fashion accessories to transplant organs and pharmaceuticals. For example, 3D printers can print medical devices with intricate designs, geometries, and features that fit a particular patient's anatomy.
3D bioprinting of organs and tissues is described in the articles " Organs from the laboratory " [1] and " Artificial organs and tissue engineering " [2]. — Ed.
Medical 3D printing is rapidly revolutionizing healthcare. The use of 3D printing in medicine brings wide-ranging benefits: personalization of medical devices, medicines, cost-effectiveness, increased productivity, and democratization of design and production.
Before we start delving into the topic of 3D printing in the pharmaceutical industry, let's figure out what 3D printing actually is.
Three-dimensional (3D) printing is an additive (additive) manufacturing method in which objects are made in layers by melting and sintering solid or solidifying liquid materials (ceramics, plastics, metals, powders, liquids, or even living cells).
There are about two dozen 3D printing methods that use different printing technologies, resolutions and speeds. There are hundreds of materials from which you can recreate a 3D object of almost any shape.
There are hundreds of materials from which you can recreate a 3D object of almost any shape.
In order for a three-dimensional object to come into being, you must first create a digital model in a 3D editor, or CAD program, and export it to STL format. Using a special slicer program, translate the STL file into a control G-code for a 3D printer, prepare the 3D printer for work, and start printing. Essential Printer Elements - working platform (it forms the object) and print head (it forms the object layer by layer). Some time passes (if the object is small - a few minutes or hours, and if it is large, then printing can take more than a day), and voila, the object is ready!
What is 3D printing we learned, move on.
A bit of history
Figure 1. The most important advances in pharmaceutical 3D printing
- Early 70s. Pierre Ciraud ( Pierre Ciraud ) described the method of applying a powder material and then solidifying each layer under the influence of a high energy beam.
- 1984 Stereolithography (SLA) , invented by Chuck Hull ( Chuck Hull ), was the first commercially available 3D printing technology. This method is based on the photopolymerization of liquid resin with ultraviolet light.
- Mid 1980s. Karl Deckard ( Carl Deckard ) developed a method for solidifying powder layers using a laser beam, which he called the Selective Laser Sintering (SLS) method.
- 1989 Scott Crump ( Skott Crump ) patented the Rapid Prototyping (FDM) technology, a method that uses a thermoplastic material to form a 3D object. Otherwise this technology is called deposition modeling .
- 1990s. Invented DOS method - basically the same as used in inkjet printers.
- 2008 Invented the RepRap printer, a self-copying mechanism for rapid prototyping.
- 2015 The American company Aprecia Pharmaceuticals has developed ZipDose technology, which allows to form tablets that are convenient because they quickly dissolve in a small amount of water.
 This technology produced the first FDA-approved printed drug, Spritam® ( Food and drug administration , US Food and Drug Administration) [3].
This technology produced the first FDA-approved printed drug, Spritam® ( Food and drug administration , US Food and Drug Administration) [3].
Dictionary
- DOD
- ( Drop On Drop ) liquid overlay.
- DOS
- ( Drop On Solid ) hard overlay.
- FDM
- fused deposition method, additive manufacturing technology.
- SLA
- stereolithography, additive manufacturing technology for models from liquid photopolymer resins.
- SLS
- selective laser sintering, additive manufacturing technology.
- STL
- file format widely used for storing 3D object models for use in additive technologies.
How drug printing works
Many different 3D printing techniques have been invented and developed over its 40 year history.
The basic 3D printing methods (fig. 2) are based on:
- solidification of the powder material
- curing fluid
- extrusion [3]
Figure 2. 3D printing methods used to create medicines
3D printing methods used to create medicines
Despite the variety of 3D printing methods, each of them includes the following steps, which we talked about at the beginning (Fig. 3) [3] :
- designing a 3D object using software and optimizing the geometry of the object according to the printer specification;
- export of a 3D model to a file format recognized by the printer, for example, STL;
- importing a file into the software and creating layers in it that will be printed. The height of the printed layer significantly affects the quality of the object, as well as the print time;
- fabrication of an object by subsequent application (or curing) of layers of material.
Figure 3. Stages of 3D printing, development
Application of 3D printing in pharmaceuticals (examples) see where these technologies are already being successfully used to create pharmaceutical products.
Example 1
As reported above, the first 3D printed drug was Spritam® (Fig. 4), developed by the American pharmaceutical company Aprecia Pharmaceuticals and approved by the Food and Drug Administration (FDA) . The active substance of the drug - levetiracetam - is an antiepileptic drug. Levetiracetam is able to quickly dissolve in the mouth, the time of disintegration (dissolution) of the drug is from 2 to 27 seconds (average - 11 seconds). A small sip of water is required to disintegrate the drug. The liquid formula, which binds levetiracetam and excipients for the manufacture of the drug, contains flavor-masking additives that improve the patient's condition [4].
4), developed by the American pharmaceutical company Aprecia Pharmaceuticals and approved by the Food and Drug Administration (FDA) . The active substance of the drug - levetiracetam - is an antiepileptic drug. Levetiracetam is able to quickly dissolve in the mouth, the time of disintegration (dissolution) of the drug is from 2 to 27 seconds (average - 11 seconds). A small sip of water is required to disintegrate the drug. The liquid formula, which binds levetiracetam and excipients for the manufacture of the drug, contains flavor-masking additives that improve the patient's condition [4].
Figure 4: Spritam® (levetiracetam), the first 3D printed drug
Aprecia Pharmaceuticals
Example 2 medical products. The company was founded by a group of scientists from University College London who saw the potential of 3D printing technology to create drugs [5].
Figures 5-7 show FabRx developments.
Figure 5. Gummy-like drugs in a variety of shapes, sizes, colors, textures and flavors. FabRx makes them attractive to different patient groups, especially young and old.
FabRx makes them attractive to different patient groups, especially young and old.
fabrx.co.uk
Figure 6. FabRx researchers experimented with the sizes and shapes of preparations and conducted a study that showed that a pyramid-shaped tablet dissolves faster in water than a cylindrical one
fabrx.co.uk
Figure 7. Scientists from University College London also experimented with different drug forms (octopus, dinosaur, cat, monkey and others) for a pediatric patient group
The future of 3D printing drugs in pharmacies is closer than you think
Few examples came out, as 3D printing is just being introduced into the pharmaceutical industry, and today only a few companies are 3D printing medicines.
Benefits and prospects of 3D printing in pharmaceuticals
Benefits
- Customization and personalization. 3D printing technologies allow custom dosage forms, release profiles and dosage for each patient.
 For example, for small patients, a tablet can be printed in the form of some cute animal of any color (Fig. 7).
For example, for small patients, a tablet can be printed in the form of some cute animal of any color (Fig. 7). - Improving cost efficiency. 3D printing will reduce production costs by reducing the use of unnecessary resources. Some drugs can be printed in forms that can be easily and conveniently delivered to the patient (Fig. 8).
- Democratization. Another feature of 3D printing is the democratization of product design and manufacturing. As the cost efficiency of 3D printing increases, the products become orders of magnitude cheaper. [6]
Figure 8. FabRx Products
fabrx.co.uk
Perspectives
In the distant future, perhaps if 3D printing develops, everyone will be able to print a drug at home. So far, this can only be a dream. But in the near future, as the researchers suggest, drugs can be printed in pharmacies and hospitals.
Can 3D drug printing replace traditional drug manufacturing technologies? No - it will require huge investments, trained employees and a number of other things. Yes, and large pharmaceutical companies can prevent the penetration of 3D printing into pharmacies and hospitals. 3D printing is unlikely to catch on in large enterprises, as 3D printers print much more slowly than pharmaceutical production machines. Another significant barrier that may prevent the widespread use of 3D printing in pharmaceuticals is the long and costly obtaining approvals from the quality control services of medicines. In addition, manufacturing regulations and government legal requirements also hinder the spread of 3D drug printing [6].
Yes, and large pharmaceutical companies can prevent the penetration of 3D printing into pharmacies and hospitals. 3D printing is unlikely to catch on in large enterprises, as 3D printers print much more slowly than pharmaceutical production machines. Another significant barrier that may prevent the widespread use of 3D printing in pharmaceuticals is the long and costly obtaining approvals from the quality control services of medicines. In addition, manufacturing regulations and government legal requirements also hinder the spread of 3D drug printing [6].
Pharmaceutical 3D printing is young and still evolving. I think that 3D printing will not be able to capture the pharmaceutical market, since Big Pharma is not going to give up. And as already mentioned, in the world there are only two companies aimed at the development of three-dimensional printing of drugs - this is the American company Aprecia Pharmaceuticals and the British company FabRx.
Conclusion
3D printing has become a useful and transformative tool in a number of different areas, including pharmaceuticals. Researchers continue to improve existing 3D printing technologies. Medical and pharmaceutical advances with the help of 3D printing are already serious and exciting, but it will take time and money for everyone to be able to come to the pharmacy and print their own drug.
Researchers continue to improve existing 3D printing technologies. Medical and pharmaceutical advances with the help of 3D printing are already serious and exciting, but it will take time and money for everyone to be able to come to the pharmacy and print their own drug.
- Laboratory organs;
- Artificial organs and tissue engineering;
- Witold Jamróz, Joanna Szafraniec, Mateusz Kurek, Renata Jachowicz. (2018). 3D Printing in Pharmaceutical and Medical Applications – Recent Achievements and Challenges. Pharm Res . 35 ;
- MurtazaM Tambuwala, NitinB Charbe, PaulA McCarron, MajellaE Lane. (2017). Application of three-dimensional printing for colon targeted drug delivery systems. Int J Pharma Investig . 7 .47;
- Abdul W. Basit, Simon Gaisford 3D Printing of Pharmaceuticals - Springer International Publishing, 2018;
- Ventola C.
 L. (2014). Medical applications for 3D printing: current and projected uses. P. T. 39 , 704–711.
L. (2014). Medical applications for 3D printing: current and projected uses. P. T. 39 , 704–711.
Comments
Pharmacy 3D printing | Remedium.ru
Magazine "Remedium" №9, 2020
DOI: 10.21518/1561-5936-2020-9-58-60
Julia Prozherina, PhD,
REM Analytics
3D printing of medicines is an innovative and cost-effective technology that is an important step towards personalized medicine. This direction can be used to develop drugs with controlled release of active substances; preparations containing combinations with fixed doses, as well as for the creation of orodispersible dosage forms. The global 3D drug market is still largely at the research stage, but is expected to grow rapidly in the next decade [1].
3D printing in pharmacy
Yuliya Prozherina, Cand. of Sci. (Bio.),
RM Analytics
3D printing of drugs is an innovative and cost-effective technology, which is a major step towards personalized medicine. This technology can be used for the development of controlled-release drugs; fixed-dose combination drugs, as well as for the creation of orodispersible dosage forms. The global 3D drug market is still largely at the research stage, but its rapid growth is expected in the coming decade [1].
This technology can be used for the development of controlled-release drugs; fixed-dose combination drugs, as well as for the creation of orodispersible dosage forms. The global 3D drug market is still largely at the research stage, but its rapid growth is expected in the coming decade [1].
FROM CARS TO DRUGS
3D printing was developed in the late 1980s at the Massachusetts Institute of Technology (MIT) as a rapid prototyping technique. Currently, it has become widespread and is actively used in industries such as automotive, healthcare (primarily in dentistry and orthopedics) and retail.
Important steps are also being taken towards the development and implementation of 3D medicines into clinical practice. By the way, issued by Therics, Inc. (Princeton, New Jersey) at 19The '94 license covers the use of 3D printing for the production of various products, including medicines (MPs). The application of these technologies in the field of drug delivery has been actively investigated, and in 2015 it was implemented in the United States. In August 2015, the first 3D-printed and FDA-approved LP was produced on an industrial scale. It was the orodispersible antiepileptic drug Spritam (levetiracetam) from Aprecia Pharmaceuticals. The creation of this drug laid the foundation for the 3D future in pharmaceuticals, demonstrating the possibilities of 3D printing for the production of complex dosage forms. Research and development in this area is still ongoing [2].
In August 2015, the first 3D-printed and FDA-approved LP was produced on an industrial scale. It was the orodispersible antiepileptic drug Spritam (levetiracetam) from Aprecia Pharmaceuticals. The creation of this drug laid the foundation for the 3D future in pharmaceuticals, demonstrating the possibilities of 3D printing for the production of complex dosage forms. Research and development in this area is still ongoing [2].
IMPORTANT ADVANTAGES
3D drug printing technology is becoming an important step in the development of personalized medicine, as it allows implementing the principle of individual selection of components and their dosage depending on the needs of the patient. In addition, when creating a dosage form (DF) according to the presented technology, it is possible to adjust the release profile of the active ingredient depending on the individual characteristics of the patient. Particularly relevant is the creation on the basis of this technology of easily and quickly soluble orodispersible drugs in the oral cavity.
3D printing of drugs may also be in demand in the orphan drug segment, since these drugs are produced in small quantities.
From a technological point of view, the advantages of additive methods in the development of dosage forms are the ability to accurately control the spatial distribution of pharmaceutical substances, create complex geometries, precipitate small amounts of pharmaceutical substances, reduce waste, and speed up the production of various compositions for screening studies or the manufacture of individualized drugs. The manufacturing benefits associated with LP printing are moving away from traditionally complex, slow and costly production chains and enabling more personalized products without the need for high volume production [2]. To some extent, 3D printing can become a continuation of the pharmacy tradition of manufacturing medicines based on individual recipes, but at a technologically new level and implemented in a slightly different way.
WORLDWIDE 3D
According to Maximize Market Research, the global 3D medicine market reached $245M in 2019 and is expected to grow to $456M by 2027 (CAGR 8 . 07%).
07%).
Key factors driving the growth of the market are innovations in 3D printing technologies, as well as an increase in diseases such as epilepsy, schizophrenia, in which the use of instantly soluble dosage forms in the oral cavity is important. On the other hand, unforeseen technological difficulties, the need for large investments in further research, the introduction of various regulation scenarios for this industry segment, as well as the likelihood of side effects of drugs manufactured by this method can hinder further market growth [1, 3].
The global 3D printed medicines market can be segmented by dosage form, manufacturing technology and geography.
Based on dosage form, the market is categorized into tablets, capsules, multi-drug implant, nanoparticles, solutions, nanosuspensions, encapsulated polymer and implant [1].
In terms of applied 3D drug manufacturing technology, the market is divided into inkjet printing, direct-write, zip dose, thermal inkjet (TIJ), deposition modeling (FDM), powder printing and stereolithography (SLA) [1]. Despite the fact that over the past 15 years a large number of different 3D printing technologies have been introduced into the LP prototyping industry, inkjet printing is still the most popular [2].
Despite the fact that over the past 15 years a large number of different 3D printing technologies have been introduced into the LP prototyping industry, inkjet printing is still the most popular [2].
Geographically, the market is divided into North America, Europe, Asia Pacific (APAC), South America, and the Middle East and Africa (MEA). The Asia-Pacific region holds the leading market share in the 3D printed medicine market, accounting for 34.1%. It is expected to act as a further growth driver for the segment (CAGR 18.2%). At the same time, countries in the Asia-Pacific region, such as China and India, will grow at the highest rates thanks to huge investments in both R&D and the pharmaceutical industry. In the European region, growth in the development of this technology is also predicted [3].
Drawing. Global 3D Printed Medicines Market, by Region: 2020-2027
Source: Maximize. Market Research PVT. LTD [3]
There are still few players in the global market for 3D printed medicines. The key ones are GlaxoSmithKline PLC and Aprecia Pharmaceuticals LLC, Fabrx Ltd [1]. Merck, Hewlett Packard Caribe, BV, LLC, 3D Printer Drug Machine, Cycle Pharmaceuticals, etc. are actively engaged in development in this direction [4]. In order to stay in the competitive market, the leading players use various strategies such as acquisitions, mergers, expansions, joint ventures and products. For example, GlaxoSmithKline PLC (GSK) has been investing in 3D printing technology for several years. The R&D department of the 3D printing company uses it in a variety of ways, from drug prototyping to drug packaging. The goal of Fabrx Ltd and GSK is to develop approaches to individualized medicine for each patient using 3D printing [1].
The key ones are GlaxoSmithKline PLC and Aprecia Pharmaceuticals LLC, Fabrx Ltd [1]. Merck, Hewlett Packard Caribe, BV, LLC, 3D Printer Drug Machine, Cycle Pharmaceuticals, etc. are actively engaged in development in this direction [4]. In order to stay in the competitive market, the leading players use various strategies such as acquisitions, mergers, expansions, joint ventures and products. For example, GlaxoSmithKline PLC (GSK) has been investing in 3D printing technology for several years. The R&D department of the 3D printing company uses it in a variety of ways, from drug prototyping to drug packaging. The goal of Fabrx Ltd and GSK is to develop approaches to individualized medicine for each patient using 3D printing [1].
Work on the creation of LP using 3D printing technology is also underway in our country. For example, scientists at the St. Petersburg Chemical and Pharmaceutical University plan to create a technology for individual drug dosages using 3D printing. The university has a Russian-made Picasso 3D printer. Another promising project in the field of bioprinting for the university is the creation of drugs with controlled release [5].
Another promising project in the field of bioprinting for the university is the creation of drugs with controlled release [5].
DRIVER – COVID
While the creation of 3D medicines still requires more research, the production of medical devices and various parapharmaceutical products is progressing more rapidly. In addition, the COVID-19 pandemic has only accelerated this process. So, for example, in many countries of the world this spring, medical products designed to protect against coronavirus were printed on 3D printers. Printed medical masks in the UK were provided by iMark, and in Russia by Temporum (a resident of the Nagatino technopark). Carbon has been actively printing face shields. In Spain, Consorci de la Zona Franca, HP Inc., Leitat and CatSalut have developed the first 3D printed emergency ventilation device. Devices printed on a 3D printer were actively purchased by hospitals in Italy [6]. And this is not the limit of the possibilities of using additive technologies. According to Forbes, the coronavirus pandemic could be a truly high point for 3D printing [7].
According to Forbes, the coronavirus pandemic could be a truly high point for 3D printing [7].
References
- 3D Printed Drugs Market Research and Forecast 2018-2027. available at: https://www.omrglobal.com/.
- Blynskaya E.V., Tishkov S.V., Alekseev K.V. 3D printing technologies for the production of dosage forms. Drug development and registration . 2018;(24):10–19.
- Global 3D Printed Drugs Market–Industry Analysis and Forecast (2020-2027) – By Scenario, End User and Region. Available at: https://www.maximizemarketresearch.com/.
- 3D Printed Drugs Market 2019 Top Key Players are 3D Printing Systems, Aprecia Pharmaceuticals, Hewlett Packard Enterprise, Hewlett Packard Enterprise, GlaxoSmithKline PLC and Forecast to 2025. Source: Available at: https://www.medgadget.com/.
- Using 3D printing technology, it is possible to control drug release in the body. Access mode: https://gxpnews.net/.
- 3D printing in medicine.

Learn more