3D printing drug delivery system
3D Printing Technology in Drug Delivery: Recent Progress and Application
Review
. 2018;24(42):5039-5048.
doi: 10.2174/1381612825666181206123828.
Sabna Kotta 1 , Anroop Nair 2 , Nimer Alsabeelah 1
Affiliations
Affiliations
- 1 College of Pharmacy and Dentistry, Buraydah Private Colleges, Buraydah, Saudi Arabia.
- 2 College of Clinical Pharmacy, King Faisal University, Al Ahsa, Saudi Arabia.
- PMID: 30520368
- DOI: 10.
2174/1381612825666181206123828
Review
Sabna Kotta et al. Curr Pharm Des. 2018.
. 2018;24(42):5039-5048.
doi: 10.2174/1381612825666181206123828.
Authors
Sabna Kotta 1 , Anroop Nair 2 , Nimer Alsabeelah 1
Affiliations
- 1 College of Pharmacy and Dentistry, Buraydah Private Colleges, Buraydah, Saudi Arabia.
- 2 College of Clinical Pharmacy, King Faisal University, Al Ahsa, Saudi Arabia.
- PMID: 30520368
- DOI: 10.
 2174/1381612825666181206123828
2174/1381612825666181206123828
Abstract
Background: 3D printing technology is a new chapter in pharmaceutical manufacturing and has gained vast interest in the recent past as it offers significant advantages over traditional pharmaceutical processes. Advances in technologies can lead to the design of suitable 3D printing device capable of producing formulations with intended drug release.
Methods: This review summarizes the applications of 3D printing technology in various drug delivery systems. The applications are well arranged in different sections like uses in personalized drug dosing, complex drugrelease profiles, personalized topical treatment devices, novel dosage forms and drug delivery devices and 3D printed polypills.
Results: This niche technology seems to be a transformative tool with more flexibility in pharmaceutical manufacturing.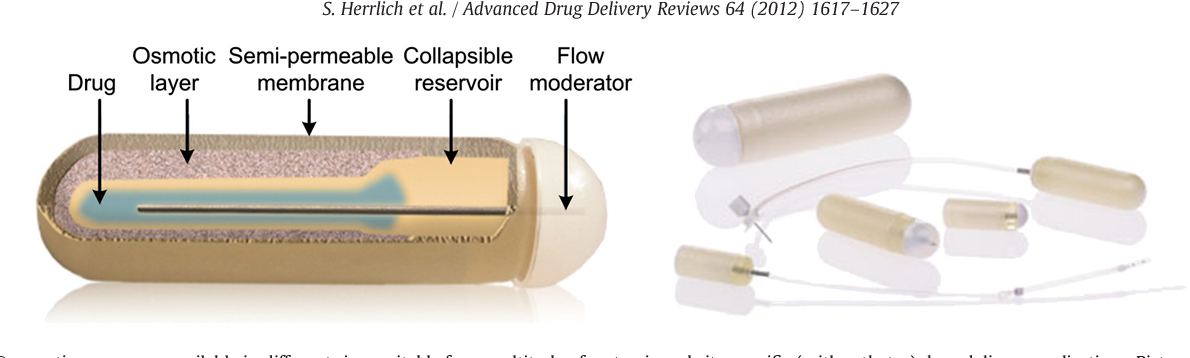 Typically, 3D printing is a layer-by-layer process having the ability to fabricate 3D formulations by depositing the product components by digital control. This additive manufacturing process can provide tailored and individualized dosing for treatment of patients different backgrounds with varied customs and metabolism pattern. In addition, this printing technology has the capacity for dispensing low volumes with accuracy along with accurate spatial control for customized drug delivery. After the FDA approval of first 3D printed tablet Spritam, the 3D printing technology is extensively explored in the arena of drug delivery.
Typically, 3D printing is a layer-by-layer process having the ability to fabricate 3D formulations by depositing the product components by digital control. This additive manufacturing process can provide tailored and individualized dosing for treatment of patients different backgrounds with varied customs and metabolism pattern. In addition, this printing technology has the capacity for dispensing low volumes with accuracy along with accurate spatial control for customized drug delivery. After the FDA approval of first 3D printed tablet Spritam, the 3D printing technology is extensively explored in the arena of drug delivery.
Conclusion: There is enormous scope for this promising technology in designing various delivery systems and provides customized patient-compatible formulations with polypills. The future of this technology will rely on its prospective to provide 3D printing systems capable of manufacturing personalized doses. In nutshell, the 3D approach is likely to revolutionize drug delivery systems to a new level, though need time to evolve.
In nutshell, the 3D approach is likely to revolutionize drug delivery systems to a new level, though need time to evolve.
Keywords: 3D printed polypill; 3D printing; fused deposition; hot-melt extrusion; personalized drug dosing; stereolithographic 3D printing..
Copyright© Bentham Science Publishers; For any queries, please email at [email protected].
Similar articles
-
3D Printing technologies for drug delivery: a review.
Prasad LK, Smyth H. Prasad LK, et al. Drug Dev Ind Pharm. 2016;42(7):1019-31. doi: 10.3109/03639045.2015.1120743. Epub 2015 Dec 13. Drug Dev Ind Pharm. 2016. PMID: 26625986 Review.
-
3D Printing Methods for Pharmaceutical Manufacturing: Opportunity and Challenges.

Warsi MH, Yusuf M, Al Robaian M, Khan M, Muheem A, Khan S. Warsi MH, et al. Curr Pharm Des. 2018;24(42):4949-4956. doi: 10.2174/1381612825666181206121701. Curr Pharm Des. 2018. PMID: 30520367 Review.
-
Pharmaceutical Product Development Exploiting 3D Printing Technology: Conventional to Novel Drug Delivery System.
Alam MS, Akhtar A, Ahsan I, Shafiq-Un-Nabi S. Alam MS, et al. Curr Pharm Des. 2018;24(42):5029-5038. doi: 10.2174/1381612825666190206195808. Curr Pharm Des. 2018. PMID: 30727872 Review.
-
A new chapter in pharmaceutical manufacturing: 3D-printed drug products.
Norman J, Madurawe RD, Moore CM, Khan MA, Khairuzzaman A. Norman J, et al. Adv Drug Deliv Rev. 2017 Jan 1;108:39-50.
 doi: 10.1016/j.addr.2016.03.001. Epub 2016 Mar 18. Adv Drug Deliv Rev. 2017. PMID: 27001902 Review.
doi: 10.1016/j.addr.2016.03.001. Epub 2016 Mar 18. Adv Drug Deliv Rev. 2017. PMID: 27001902 Review. -
3D Printing Technologies: Recent Development and Emerging Applications in Various Drug Delivery Systems.
Jacob S, Nair AB, Patel V, Shah J. Jacob S, et al. AAPS PharmSciTech. 2020 Aug 3;21(6):220. doi: 10.1208/s12249-020-01771-4. AAPS PharmSciTech. 2020. PMID: 32748243 Review.
See all similar articles
Cited by
-
Clinical Application of Digital 3D Reconstruction and 3D Printing Technology in Endometrial Cancer (EC) Surgery.
Luo F, Yang Q. Luo F, et al. Comput Math Methods Med. 2022 Sep 14;2022:9180216. doi: 10.1155/2022/9180216. eCollection 2022.
 Comput Math Methods Med. 2022. PMID: 36158121 Free PMC article.
Comput Math Methods Med. 2022. PMID: 36158121 Free PMC article. -
The Evolution of the 3D-Printed Drug Delivery Systems: A Review.
Bácskay I, Ujhelyi Z, Fehér P, Arany P. Bácskay I, et al. Pharmaceutics. 2022 Jun 21;14(7):1312. doi: 10.3390/pharmaceutics14071312. Pharmaceutics. 2022. PMID: 35890208 Free PMC article. Review.
-
Design Aspects of Additive Manufacturing at Microscale: A Review.
Rogkas N, Vakouftsis C, Spitas V, Lagaros ND, Georgantzinos SK. Rogkas N, et al. Micromachines (Basel). 2022 May 15;13(5):775. doi: 10.3390/mi13050775. Micromachines (Basel). 2022. PMID: 35630242 Free PMC article. Review.
-
Sensing and 3D printing technologies in personalized healthcare for the management of health crises including the COVID-19 outbreak.
Kalkal A, Allawadhi P, Kumar P, Sehgal A, Verma A, Pawar K, Pradhan R, Paital B, Packirisamy G. Kalkal A, et al. Sens Int. 2022;3:100180. doi: 10.1016/j.sintl.2022.100180. Epub 2022 May 14. Sens Int. 2022. PMID: 35601184 Free PMC article. Review.
-
Alginate Self-Crosslinking Ink for 3D Extrusion-Based Cryoprinting and Application for Epirubicin-HCl Delivery on MCF-7 Cells.
Remaggi G, Catanzano O, Quaglia F, Elviri L. Remaggi G, et al. Molecules. 2022 Jan 27;27(3):882. doi: 10.3390/molecules27030882. Molecules. 2022. PMID: 35164146 Free PMC article.
See all "Cited by" articles
Publication types
MeSH terms
3D Printing technologies for drug delivery: a review
Review
. 2016;42(7):1019-31.
2016;42(7):1019-31.
doi: 10.3109/03639045.2015.1120743. Epub 2015 Dec 13.
Leena Kumari Prasad 1 , Hugh Smyth 1
Affiliations
Affiliation
- 1 a Division of Pharmaceutics , College of Pharmacy, University of Texas at Austin , Austin , TX , USA.
- PMID: 26625986
- DOI: 10.3109/03639045.2015.1120743
Review
Leena Kumari Prasad et al. Drug Dev Ind Pharm. 2016.
. 2016;42(7):1019-31.
doi: 10.3109/03639045.2015.1120743. Epub 2015 Dec 13.
Authors
Leena Kumari Prasad 1 , Hugh Smyth 1
Affiliation
- 1 a Division of Pharmaceutics , College of Pharmacy, University of Texas at Austin , Austin , TX , USA.
- PMID: 26625986
- DOI: 10.3109/03639045.2015.1120743
Abstract
With the FDA approval of the first 3D printed tablet, Spritam®, there is now precedence set for the utilization of 3D printing for the preparation of drug delivery systems. The capabilities for dispensing low volumes with accuracy, precise spatial control and layer-by-layer assembly allow for the preparation of complex compositions and geometries. The high degree of flexibility and control with 3D printing enables the preparation of dosage forms with multiple active pharmaceutical ingredients with complex and tailored release profiles. A unique opportunity for this technology for the preparation of personalized doses to address individual patient needs. This review will highlight the 3D printing technologies being utilized for the fabrication of drug delivery systems, as well as the formulation and processing parameters for consideration. This article will also summarize the range of dosage forms that have been prepared using these technologies, specifically over the last 10 years.
The capabilities for dispensing low volumes with accuracy, precise spatial control and layer-by-layer assembly allow for the preparation of complex compositions and geometries. The high degree of flexibility and control with 3D printing enables the preparation of dosage forms with multiple active pharmaceutical ingredients with complex and tailored release profiles. A unique opportunity for this technology for the preparation of personalized doses to address individual patient needs. This review will highlight the 3D printing technologies being utilized for the fabrication of drug delivery systems, as well as the formulation and processing parameters for consideration. This article will also summarize the range of dosage forms that have been prepared using these technologies, specifically over the last 10 years.
Keywords: 3D printing; drug delivery; fused deposition modeling; inkjet powder bed printing; particle printing; personalized dosage forms; tablets.
Similar articles
-
Emergence of 3D Printed Dosage Forms: Opportunities and Challenges.
Alhnan MA, Okwuosa TC, Sadia M, Wan KW, Ahmed W, Arafat B. Alhnan MA, et al. Pharm Res. 2016 Aug;33(8):1817-32. doi: 10.1007/s11095-016-1933-1. Epub 2016 May 18. Pharm Res. 2016. PMID: 27194002 Review.
-
3D Printing Technology in Drug Delivery: Recent Progress and Application.
Kotta S, Nair A, Alsabeelah N. Kotta S, et al. Curr Pharm Des. 2018;24(42):5039-5048. doi: 10.2174/1381612825666181206123828. Curr Pharm Des. 2018. PMID: 30520368 Review.
-
3D Printing of Solid Oral Dosage Forms: Numerous Challenges With Unique Opportunities.

Okafor-Muo OL, Hassanin H, Kayyali R, ElShaer A. Okafor-Muo OL, et al. J Pharm Sci. 2020 Dec;109(12):3535-3550. doi: 10.1016/j.xphs.2020.08.029. Epub 2020 Sep 23. J Pharm Sci. 2020. PMID: 32976900 Review.
-
3D Printing Technologies: Recent Development and Emerging Applications in Various Drug Delivery Systems.
Jacob S, Nair AB, Patel V, Shah J. Jacob S, et al. AAPS PharmSciTech. 2020 Aug 3;21(6):220. doi: 10.1208/s12249-020-01771-4. AAPS PharmSciTech. 2020. PMID: 32748243 Review.
-
3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets.
Goyanes A, Buanz AB, Hatton GB, Gaisford S, Basit AW. Goyanes A, et al. Eur J Pharm Biopharm. 2015 Jan;89:157-62.
 doi: 10.1016/j.ejpb.2014.12.003. Epub 2014 Dec 9. Eur J Pharm Biopharm. 2015. PMID: 25497178
doi: 10.1016/j.ejpb.2014.12.003. Epub 2014 Dec 9. Eur J Pharm Biopharm. 2015. PMID: 25497178
See all similar articles
Cited by
-
3D printing of pharmaceuticals: approach from bench scale to commercial development.
Pawar R, Pawar A. Pawar R, et al. Futur J Pharm Sci. 2022;8(1):48. doi: 10.1186/s43094-022-00439-z. Epub 2022 Nov 26. Futur J Pharm Sci. 2022. PMID: 36466365 Free PMC article. Review.
-
Three-Dimensional Printing Technologies for Drug Delivery Applications: Processes, Materials, and Effects.
Mancilla-De-la-Cruz J, Rodriguez-Salvador M, An J, Chua CK. Mancilla-De-la-Cruz J, et al. Int J Bioprint. 2022 Oct 20;8(4):622. doi: 10.18063/ijb.v8i4.
 622. eCollection 2022. Int J Bioprint. 2022. PMID: 36404786 Free PMC article. Review.
622. eCollection 2022. Int J Bioprint. 2022. PMID: 36404786 Free PMC article. Review. -
Polymers in Technologies of Additive and Inkjet Printing of Dosage Formulations.
Blynskaya EV, Tishkov SV, Alekseev KV, Vetcher AA, Marakhova AI, Rejepov DT. Blynskaya EV, et al. Polymers (Basel). 2022 Jun 22;14(13):2543. doi: 10.3390/polym14132543. Polymers (Basel). 2022. PMID: 35808591 Free PMC article. Review.
-
Design Aspects of Additive Manufacturing at Microscale: A Review.
Rogkas N, Vakouftsis C, Spitas V, Lagaros ND, Georgantzinos SK. Rogkas N, et al. Micromachines (Basel). 2022 May 15;13(5):775. doi: 10.3390/mi13050775. Micromachines (Basel). 2022. PMID: 35630242 Free PMC article. Review.

-
Printing Technologies as an Emerging Approach in Gas Sensors: Survey of Literature.
Simonenko NP, Fisenko NA, Fedorov FS, Simonenko TL, Mokrushin AS, Simonenko EP, Korotcenkov G, Sysoev VV, Sevastyanov VG, Kuznetsov NT. Simonenko NP, et al. Sensors (Basel). 2022 May 3;22(9):3473. doi: 10.3390/s22093473. Sensors (Basel). 2022. PMID: 35591162 Free PMC article. Review.
See all "Cited by" articles
Publication types
MeSH terms
Substances
STM Magazine - Article View
- About
- Editorial Board
- Editorial Board
- Review procedure
- How to write an article
- Requirements for Manuscripts
- Uniform International Requirements
- Magazine subscription
- Contacts
- Log archive
- Conferences
- Submit an article to journal
Site search
- Abstract
- References
- How to cite in References
In our latest methodological paper (Senkov et al. , 2015, Frontiers in Neuroscience), we presented a versatile, reusable hybrid infusion recording system (GIRS) that can be used to test freely roaming mice performing cognitive tasks with simultaneous electrophysiological recording of their brain activity in terms of local field potential and with resolution for each electrode. The system can be combined with drug delivery into the cerebral cortex and hippocampus several hours before behavioral experiments. In this paper, we describe an improved version of the GIRS system, in which some parts can be quickly produced using modern 3D printing technology. Our preliminary results show that the use of 3D printers to manufacture small parts of the system facilitates and speeds up the entire manufacturing process and improves the accuracy and reliability of GIRS implants. In addition, the wear resistance and safety of implants are increased, which contributes to the normal state of mice in the postoperative period and during long-term behavioral tests.
, 2015, Frontiers in Neuroscience), we presented a versatile, reusable hybrid infusion recording system (GIRS) that can be used to test freely roaming mice performing cognitive tasks with simultaneous electrophysiological recording of their brain activity in terms of local field potential and with resolution for each electrode. The system can be combined with drug delivery into the cerebral cortex and hippocampus several hours before behavioral experiments. In this paper, we describe an improved version of the GIRS system, in which some parts can be quickly produced using modern 3D printing technology. Our preliminary results show that the use of 3D printers to manufacture small parts of the system facilitates and speeds up the entire manufacturing process and improves the accuracy and reliability of GIRS implants. In addition, the wear resistance and safety of implants are increased, which contributes to the normal state of mice in the postoperative period and during long-term behavioral tests.
- Senkov O., Mironov A., Dityatev A. A novel versatile hybrid infusion-multielectrode recording (HIME) system for acute drug delivery and multisite acquisition of neuronal activity in freely moving mice. Front Neurosci 2015; 9:425, https://doi.org/10.3389/fnins.2015.00425.
- Wlodarczyk J., Mukhina I., Kaczmarek L., Dityatev A. Extracellular matrix molecules, their receptors, and secreted proteases in synaptic plasticity. Dev Neurobiol 2011; 71(11): 1040–1053, https://doi.org/10.1002/dneu.20958.
- Kochlamazashvili G., Senkov O., Grebenyuk S., Robinson C., Xiao M.F., Stummeyer K., Gerardy-Schahn R., Engel A.K., Feig L., Semyanov A., Suppiramaniam V., Schachner M., Dityatev A. Neural cell adhesion molecule-associated polysialic acid regulates synaptic plasticity and learning by restraining the signaling through GluN2B-containing NMDA receptors. J Neurosci 2010; 30(11): 4171–4183, https://doi.org/10.1523/JNEUROSCI.5806-09.2010.
- Pizzorusso T., Medini P.
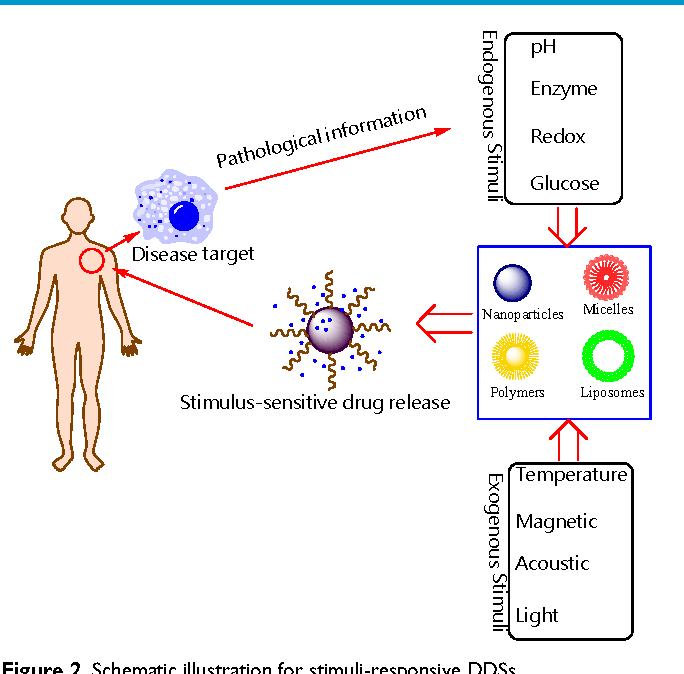 , Landi S., Baldini S., Berardi N., Maffei L. Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci USA 2006; 103(22): 8517–8522, https://doi.org/10.1073/pnas.0602657103.
, Landi S., Baldini S., Berardi N., Maffei L. Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci USA 2006; 103(22): 8517–8522, https://doi.org/10.1073/pnas.0602657103. - Korotchenko S., Cingolani L.A., Kuznetsova T., Bologna L.L., Chiappalone M., Dityatev A. Modulation of network activity and induction of homeostatic synaptic plasticity by enzymatic removal of heparan sulfates. Philos Trans R Soc Lond B Biol Sci 2014; 369(1654): 20140134, https://doi.org/10.1098/rstb.2014.0134.
- Dityatev A., Frischknecht R., Seidenbecher C.I. Extracellular matrix and synaptic functions. Results Probl Cell Differ 2006; 43: 69–97, https://doi.org/10.1007/400_025.
- Senkov O., Andjus P., Radenovic L., Soriano E., Dityatev A. Neural ECM molecules in synaptic plasticity, learning, and memory. Prog Brain Res 2014; 214: 53–80, https://doi.org/10.1016/B978-0-444-63486-3.00003-7.
Senkov O. , Mironov A., Dityatev A. An Advanced 3D Printed Design of the Hybrid Infusion-Multielectrode Recording System for Local Field Potential and Single Unit Acquisition and Intrabrain Drug Delivery in Freely Moving Mice. Modern technologies v medicine 2016; 8(4): 129, https://doi.org/10.17691/stm2016.8.4.17
, Mironov A., Dityatev A. An Advanced 3D Printed Design of the Hybrid Infusion-Multielectrode Recording System for Local Field Potential and Single Unit Acquisition and Intrabrain Drug Delivery in Freely Moving Mice. Modern technologies v medicine 2016; 8(4): 129, https://doi.org/10.17691/stm2016.8.4.17
Researchers 3D print hollow microneedles for controlled transdermal drug delivery
University of Kent and Strathclyde researchers have developed a new device that combines 3D microneedles and micromechanical printing systems (MEMS) for controlled transdermal drug delivery.
Transdermal drug delivery refers to the application of a drug or drug through the skin, usually using an adhesive patch. For their method, the researchers developed a composite device consisting of a 3D printed patch with microneedles combined with MEMS, allowing the user to directly control the administration of drugs.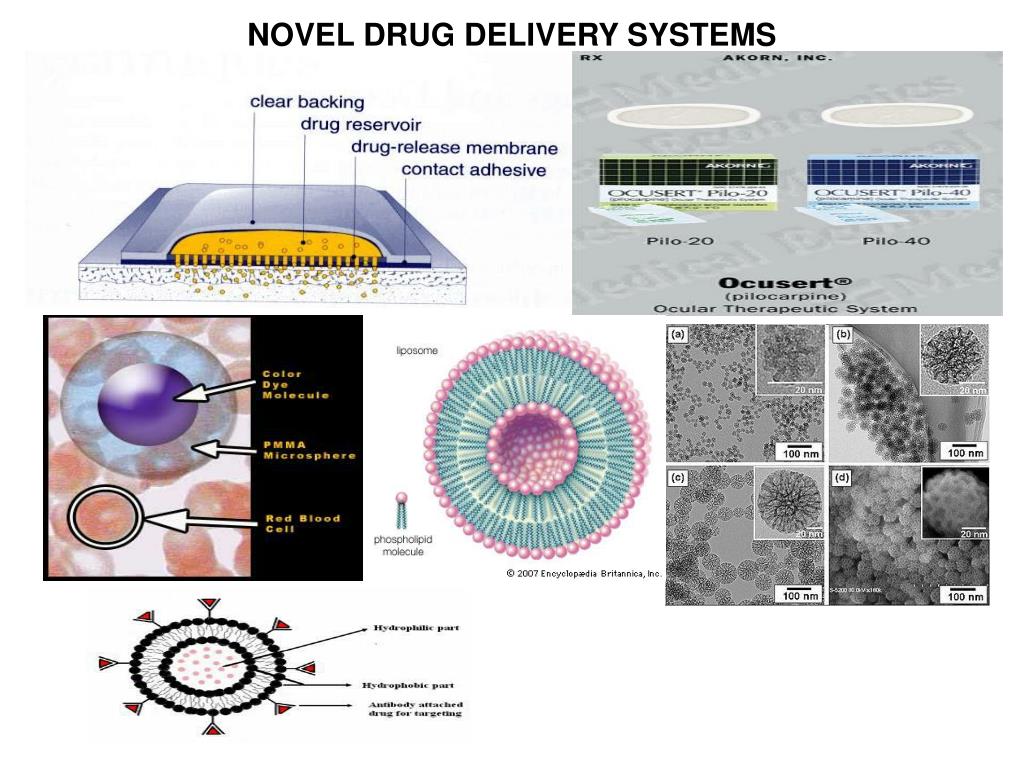
Called 3DMNMEMS, the device was designed with one goal in mind - to personalize clinical care and make generic drug delivery a thing of the past.
"Microneedles are small piercing devices that puncture the outermost, most impenetrable layer of the skin to transport drugs directly into the skin's microcirculation environment," said Sophia Economidou, one of the researchers at the University of Kent. “Their miniature size means they are painless to use and solve problems such as patient discomfort and needle phobia.
"THE MICRONEEDLE TRANSDERMAL SYSTEMS ALSO ARE SELF-MANAGED WITHOUT THE NEED FOR THE INTERVENTION OF A TRAINED PROFESSIONAL, REDUCING TREATMENT COSTS."
Typically, conventional microneedles are produced by micro-casting, a process that is difficult to set up and involves high initial tooling costs. The researchers also noted that the fabrication of internal microstructures within their own microneedles was not possible using the micromolding technique.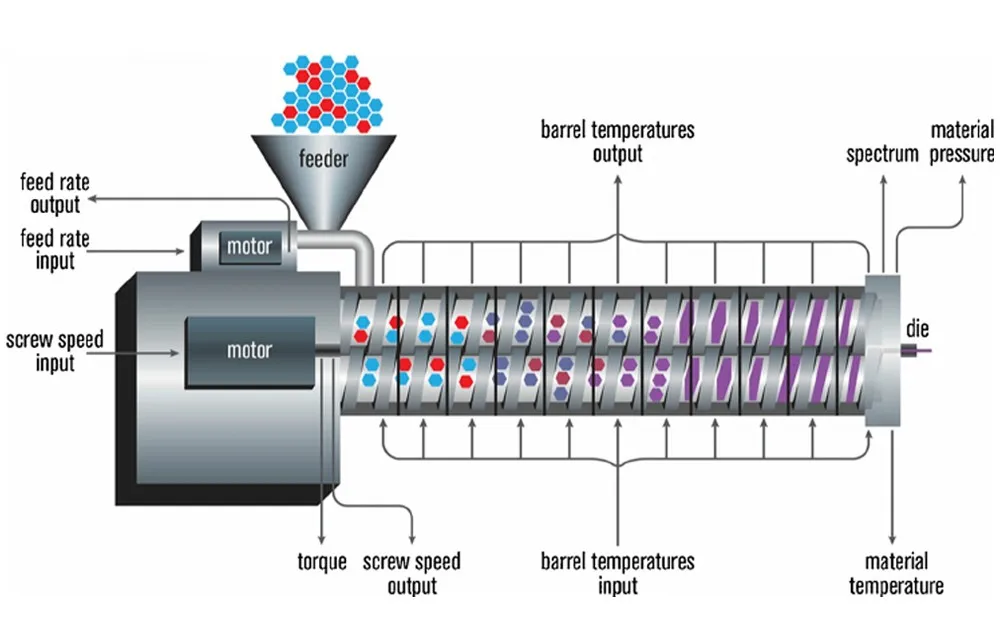
So the researchers turned to 3D printing to enable one-step, repeatable and reproducible fabrication of the complex 3DMNMEMS device. In addition, 3D printing makes it easy to customize the device, while the manufacturer can tailor the design to the individual needs of the patient. Microneedle patches can also be made to order, eliminating the need for storage space in clinics and laboratories.
Research in this area has been done before, for example, in 2018, scientists at the University of Texas at Dallas developed a new low-cost method for manufacturing microneedle arrays using an FFF 3D printer. Since then, researchers at Rutgers University have used projection microstereolithography to create 4D printed bioinspired programmable microneedles that improve tissue adhesion.
More recently, a team from Arizona State University and the University of Southern California has developed 3D printed microneedle patches inspired by the hierarchical structure of saucers. The patches are produced using magnetic field 3D printing (MF-3DP), and in the future they could be used for painless drug delivery to patients.
The patches are produced using magnetic field 3D printing (MF-3DP), and in the future they could be used for painless drug delivery to patients.
Microneedle patch, designed using standard CAD software, consists of hollow microneedle rods with holes, internal microchannels, microreservoir and fluid inlet. Microneedles are 3D printed using stereolithography and a biocompatible polymer, but researchers have found that their complex structure and the need for precise, reproducible prints challenge the capabilities of a 3D printer.
In particular, they noted the difficulty of achieving the required sharpness of the tip and ensuring that the internal microstructures were free of blockages that could restrict fluid flow.
“At the micro scale, the influence of print parameters on the quality of the final product is enhanced, so we had to develop and apply a number of optimization steps, including adjusting print parameters and adapting the design to achieve the desired result and create a reproducible print protocol,” explained Economou. "It's worth noting that the patch was designed to be easy to install on the MEMS used here, as well as on a conventional syringe."
"It's worth noting that the patch was designed to be easy to install on the MEMS used here, as well as on a conventional syringe."
To study how effective the 3DMNMEMS device was in vivo - in vivo - the researchers used it to administer insulin to diabetic mice. They then compared this group of mice with another group injected with the same amount of insulin under the skin.
According to Economoud, 3DMNMEMS provided a more rapid reduction in blood glucose (one hour), while an injection took three hours to produce the same hypoglycemic effect. The results also showed that the insulin concentration in the device group was more stable over time as a result of the wide distribution of the drug by the microneedles into the skin tissue.
“Injections typically create a depot in the skin from which the drug enters the bloodstream via passive diffusion,” Economou explained. “This leads to a delay between the intake and the peak of the effect. Microneedles, on the other hand, distribute the drug into the tissues, allowing for faster and more sustained absorption."
Microneedles, on the other hand, distribute the drug into the tissues, allowing for faster and more sustained absorption."
Most 3D printed hollow microneedles are produced by two photon polymerization (2PP), and these 3D printers tend to be expensive and have low print volumes. The 2PP process can also take a long time.
To overcome these problems, researchers' microneedle patch can be successfully printed on a commercial desktop printer, making the manufacturing process more accessible to researchers and interested companies. This, Economoud believes, will help expand their technology for use as a viable and sustainable method for producing microneedles.
“We also reported for the first time the combination of 3D printing with MEMS into a drug delivery platform device,” she said. “This achievement paves the way for a modified explanation of medical devices; Combining 3D printing with other sophisticated technologies is key to developing new devices that advance therapies and improve patients' lives. "
"
In their trials, the researchers demonstrated a controlled treatment for diabetes, although the device was designed as a versatile delivery platform for multiple drugs.
“3D printing of medical devices and drug delivery devices is still in its infancy,” added Economou. “We believe that this technology still has a lot of untapped potential for significant changes in modern clinical practice. In our group, we are working a lot on transdermal systems, drug-eluting stents, pills and dressings designed with 3D printing.”
Economou and colleagues plan to further expand their research on integrating MEMS and sensors into 3D printed medical devices to provide sophisticated personal care solutions.
More information about 3D printed microneedles can be found in the article titled "A New 3D Printed Microelectromechanical Hollow Microneedle System for Controlled Personalized Transdermal Drug Delivery" published in Additive Manufacturing.