3D printing bone grafts
A Review of 3D Printed Bone Implants
1. Deepak K., Bikramjit B., Ashutosh K.D. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials. 2020;258:120280. [PubMed] [Google Scholar]
2. Florencio S.R., Sasso G.R.D.S., Sasso-Cerri E., Simões M.J., Cerri P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. Biomed. Res. Int. 2015;2015:421746. doi: 10.1155/2015/421746. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
3. Baldwin P., Li D.J., Auston D.A., Mir H.S., Yoon R.S., Koval K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. Orthop. Trauma. 2019;33:203–213. doi: 10.1097/BOT.0000000000001420. [PubMed] [CrossRef] [Google Scholar]
4. Sohn H.-S., Oh J.K. Review of Bone Graft and Bone Substitutes with an Emphasis on Fracture Surgeries. Biomater. Res. 2019;23:9. doi: 10.1186/s40824-019-0157-y. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
5. Zhang D.W., Wu X.X., Chen J.D., Lin K.L. The Development of Collagen Based Composite Scaffolds for Bone Regeneration. Bioact. Mater. 2018;3:129–138. doi: 10.1016/j.bioactmat.2017.08.004. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
6. Renata G.R., Vanessa M.S., Thais L.D.A.M., Beatriz R.C.D.M., Cristiane S., Douglas M.G.L., Gilmar P.T. Current Advances in Bone Tissue Engineering Concerning Ceramic and Bio-glass Scaffolds: A Review. Ceram. Int. 2019;45:21051–21061. [Google Scholar]
7. Ma X.K., Yang R., Kang N., Wang R. Research on Polylactic Acid Composites Based on 3D Printing Technology. J. Jilin Inst. Chem. Technol. 2022;39:10–14. [Google Scholar]
8. Lipskas J., Deep K., Yao W. Robotic Assisted 3D Bio-printing for Repairing Bone and Cartilage Defects through a Minimally Invasive Approach. Sci. Rep. 2019;9:3746. doi: 10.1038/s41598-019-38972-2. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
9. Touri M., Kabirian F., Saadati M., Ramkrishna S., Mozafari M. Additive Manufacturing of Biomaterials—The Evolution of Rapid Prototyping. Adv. Eng. Mater. 2019;21:1800511. doi: 10.1002/adem.201800511. [CrossRef] [Google Scholar]
Touri M., Kabirian F., Saadati M., Ramkrishna S., Mozafari M. Additive Manufacturing of Biomaterials—The Evolution of Rapid Prototyping. Adv. Eng. Mater. 2019;21:1800511. doi: 10.1002/adem.201800511. [CrossRef] [Google Scholar]
10. Shivakalyani A., Nandini D., Anindita L., Chandra S.S., Seeram R., Mudrika K. Three Dimensional Bioprinting for Bone Tissue Regeneration. Curr. Opin. Biomed. Eng. 2017;2:22–28. doi: 10.1016/j.cobme.2017.03.005. [CrossRef] [Google Scholar]
11. Christy P.N., Basha S.K., Kumari V.S., Bashir A.K.H., Maaza M., Kaviyarasu K., Mariad V.A., Naif A.A., Savarimuthu I. Biopolymeric Nanocomposite Scaffolds for Bone Tissue Engineering Applications: A review. J. Drug Deliv. Sci. Technol. 2020;55:01452. doi: 10.1016/j.jddst.2019.101452. [CrossRef] [Google Scholar]
12. Sreenivasan D., Tu P.T., Dickinson M., Watson M., Blais A., Das R., Cornish J., Fernandez J. Computer Modelling Integrated with Micro-CT and Material Testing Provides Additional Insight to Evaluate Bone Treatments: Application to a Beta-glycan Derived whey Protein Mice Model. Comput. Biol. Med. 2016;68:9–20. doi: 10.1016/j.compbiomed.2015.10.017. [PubMed] [CrossRef] [Google Scholar]
Comput. Biol. Med. 2016;68:9–20. doi: 10.1016/j.compbiomed.2015.10.017. [PubMed] [CrossRef] [Google Scholar]
13. Hossein J., Bengi Y., Zafer E. A Review of Bio-ceramic Porous Scaffolds for Hard Tissue Applications: Effects of Structural Features. Ceram. Int. 2020;46:15725–15739. [Google Scholar]
14. Li X.Y., Song C.W., Fei Q., Zhang H.Y., Lin J.S., Meng H., Yang Y. Research Progress of 3D Printing Titanium Alloy Scaffold Materials for Bone Repair. J. Clin. Exp. Med. 2019;18:222–225. [Google Scholar]
15. Li Y.G. Research Progress of Biodegradable Magnesium Alloy Biomaterials in Orthopedics. Aging Appl. Synth. Mater. 2021;50:155–158. [Google Scholar]
16. Januariyasa I.K., Ika D.A., Yusril Y. Nanofibrous Poly (vinyl alcohol)/Chitosan Contained Carbonated Hydroxyapatite Nanoparticles Scaffold for Bone Tissue Engineering. Mater. Sci. Eng. C. 2020;107:110347. doi: 10.1016/j.msec.2019.110347. [PubMed] [CrossRef] [Google Scholar]
17. Hou D., Chen X.S., Mao Y.Y., Zhang Q. Research Progress of β-TCP Material Modification and its Application in Bone Defect Repair. Orthop. Biomech. Mater. Clin. Study. 2021;18:74–77. [Google Scholar]
Research Progress of β-TCP Material Modification and its Application in Bone Defect Repair. Orthop. Biomech. Mater. Clin. Study. 2021;18:74–77. [Google Scholar]
18. Kargozar S., Montazerian M., Fiume E., Baino F. Multiple and Promising Applications of Strontium (Sr)-Containing Bioactive Glasses in Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2019;7:161. doi: 10.3389/fbioe.2019.00161. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
19. Tan G.Z., Tu X.R., Guo L.Y., Zhong J.L., Zhang Y., Jiang Q.Z. Biosafety Evaluation of 3D Printed Gelatin/Sodium Alginate /58S Bioglass Scaffold for Bone Defect Repair. Chin. Tissue Eng. Res. 2022;26:521–527. [Google Scholar]
20. Hu K., Wang K.L., Cui Y.Z., Xiao Y.H., Yang G.J., Sun W.W., Wang J.D., Wang H.B., Guo L.X.Z., Han L., et al. Study on Degradation and Biological Performance of 3D Printed Mineralized Collagen-based Bone Prosthesis. Digit. Print. 2021;3:147–157. [Google Scholar]
21. Laura G., Emanuela A., Rosasilvia R. , Walter Bonani M.B., Gina L., Brunella G., Antonella M., Francesco G. Hydrogen Sulfide Releasing Silk Fibroin Scaffold for Bone Tissue Engineering. Mater. Sci. Eng. C. 2019;102:471–482. doi: 10.1016/j.msec.2019.04.039. [PubMed] [CrossRef] [Google Scholar]
, Walter Bonani M.B., Gina L., Brunella G., Antonella M., Francesco G. Hydrogen Sulfide Releasing Silk Fibroin Scaffold for Bone Tissue Engineering. Mater. Sci. Eng. C. 2019;102:471–482. doi: 10.1016/j.msec.2019.04.039. [PubMed] [CrossRef] [Google Scholar]
22. Pooyan M., Ghareib W., Ali F.D.S., Wafa I., Abdel F., Assunta B. Hyaluronic Acid/Corn Silk Extract Based Injectable NanoComposite: A Biomimetic Antibacterial Scaffold for Bone Tissue Regeneration. Mater. Sci. Eng. C. 2020;107:110192. [PubMed] [Google Scholar]
23. Tao F.H., Cheng Y.X., Shi X.W., Zheng H.F., Du Y.M., Wei X., Deng H.B. Applications of Chitin and Chitosan Nanofibers in Bone Regenerative Engineering. Carbohydr. Polym. 2020;230:115658. doi: 10.1016/j.carbpol.2019.115658. [PubMed] [CrossRef] [Google Scholar]
24. Ashwin B., Abinaya B., Prasith T.P., Chandran S.V., Yadav L.R., Vairamani M., Patil S., Selvamurugan N. 3D-Poly (Lactic Acid) Scaffolds Coated with Gelatin and Mucic Acid for Bone Tissue Engineering. Int. J. Biol. Macromol. 2020;162:523–532. doi: 10.1016/j.ijbiomac.2020.06.157. [PubMed] [CrossRef] [Google Scholar]
Int. J. Biol. Macromol. 2020;162:523–532. doi: 10.1016/j.ijbiomac.2020.06.157. [PubMed] [CrossRef] [Google Scholar]
25. Donnaloja F., Jacchetti E., Soncini M., Raimondi M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers. 2020;12:905. doi: 10.3390/polym12040905. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
26. Wang W.G., Junior J.R.P., Paulo R.L.N., David M., Jillian C., Fernanda M., Guilherme F.C., Paulo B. Engineered 3D Printed Poly(ɛ-caprolactone)/Graphene Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C. 2019;100:759–770. doi: 10.1016/j.msec.2019.03.047. [PubMed] [CrossRef] [Google Scholar]
27. Majid T.D., Mehdi A., Batoul H.B. Fabrication and Characterization of Porous Co–Cr–Mo/58S Bio-glass Nano Composite by Using Nh5HCO3 as Space holder. Mater. Des. 2015;88:406–413. [Google Scholar]
28. Zhou M., Cheng Y., Zhou X.C., Li M., Wei C.B., Zheng Y.F., Xiu P., Cai H., Liu Z.J., Wang C.M., et al. Titanium alloy medical implants based on additive manufacturing technology. Chin. Sci. Tech. Sci. 2016;46:1097–1115. [Google Scholar]
Chin. Sci. Tech. Sci. 2016;46:1097–1115. [Google Scholar]
29. Wen P., Qin Y., Chen Y. Laser. Additive Manufacturing of Zn Porous Scaffolds; Shielding Gas Flow, Surface Quality and Densification. J. Mater. Sci. Technol. 2019;35:368–376. doi: 10.1016/j.jmst.2018.09.065. [CrossRef] [Google Scholar]
30. Wang X.J., Xu S.Q., Zhou S.W. Topological Design and Additive Manufacturing of Porous Metals for Bone Scaffolds and Orthopaedic Implants: A Review. Biomaterial. 2016;83:127–141. doi: 10.1016/j.biomaterials.2016.01.012. [PubMed] [CrossRef] [Google Scholar]
31. You J., Fang L.H., Zhang Q., Gao Y.L., Peng W. Study on Osseointegration of Surface Porous Titanium Multi-Tooth Implant Based on SLM Technology. Chin. J. Biomed. Eng. 2015;34:315–322. [Google Scholar]
32. Alberto C.M., Luca L., Forleo D.M., Staffieri F., Francioso E., Di Meo A., Becerra J., Crovace A., Santos-Ruiz L. 3D Biomimetic Porous Titanium (Ti6Al4V ELI) Scaffolds for Large Bone Critical Defect Reconstruction: An Experimental Study in Sheep. Animals. 2020;10:1389. [PMC free article] [PubMed] [Google Scholar]
Animals. 2020;10:1389. [PMC free article] [PubMed] [Google Scholar]
33. Shi Y.L., Guo Z.M., Li X.M., Qi C.F., Yang X.C. Study on Preparation of Cellular Ti6Al4V Porous Titanium Skeleton by 3D Printing-Gel Casting. Powder Metall. Ind. 2018;28:34–39. [Google Scholar]
34. Zhang C.K. Master’s Thesis. Army Medical University; Chongqing, China: 2020. Preparation of 3D printed Ti6Al4V-4Cu Alloy Material and its Osteogenic Properties. [Google Scholar]
35. Xu W., Tian J.J., Liu Z., Lu X., Muhammad D.H., Yu Y., Zhou L., Qu X.H., Wen C. Novel Porous Ti35Zr28Nb Scaffolds Fabricated by Powder Metallurgy with Excellent Osteointegration Ability for Bone Tissue Engineering Applications. Mater. Sci. Eng. C. 2019;105:110015. doi: 10.1016/j.msec.2019.110015. [PubMed] [CrossRef] [Google Scholar]
36. Wang S., Liu L.L., Li K., Zhu L.H., Chen J., Hao Y.Q. Pore Functionally Graded Ti6Al4V Scaffolds for Bone Tissue Engineering Application. Mater. Des. 2019;168:107643. doi: 10.1016/j.matdes.2019.107643. [CrossRef] [Google Scholar]
Mater. Des. 2019;168:107643. doi: 10.1016/j.matdes.2019.107643. [CrossRef] [Google Scholar]
37. Shi X., Wu X.Z., Ma X.S., Liu Y., He P., Luo Y.F. Medical Application and Biocompatibility Mechanism of Tantalum. Funct. Mater. 2019;50:12001–12006. [Google Scholar]
38. Yin B.Z., Qin Y., Wen P., Zheng Y.F., Tian Y. Preparation of Metal Bone Implants by Laser Powder Bed Melting. China Laser. 2020;47:8–25. [Google Scholar]
39. Wauthle R., Johan V.D.S., Amin Y.S. Additively Manufactured Porous Tantalum Implants. Acta Biomater. 2015;14:217–225. doi: 10.1016/j.actbio.2014.12.003. [PubMed] [CrossRef] [Google Scholar]
40. Zheng Y.F., Gu X.N., Witte F. Biodegradable Metals. Mater. Sci. Eng. R. 2014;77:1–34. doi: 10.1016/j.mser.2014.01.001. [CrossRef] [Google Scholar]
41. Liu Y., Cheng Y.F., Chen X.H., Yang J.-A., Pan H., Chen D., Wang L., Zhang J., Zhu D., Wu S.L., et al. Fundamental theory of biodegradable metals—Definition, criteria, and design. Adv. Funct. Mater. 2019;29:180502. doi: 10.1002/adfm.201805402. [CrossRef] [Google Scholar]
doi: 10.1002/adfm.201805402. [CrossRef] [Google Scholar]
42. Zheng Y.F., Wu Y.H. Medical Metallic Materials in Transition. J. Metall. 2017;53:257–297. [Google Scholar]
43. Chou D.T., Wells D., Hong D., Lee B., Kuhn H., Kumta P.N. Novel Processing of Iron Manganese Alloy Based Biomaterials by Inkjet 3D Printing. Acta Biomater. 2013;9:8593–8603. doi: 10.1016/j.actbio.2013.04.016. [PubMed] [CrossRef] [Google Scholar]
44. Bose S., Banerjee D., Robertson S., Sahar V. Enhanced In Vivo Bone and Blood Vessel Formation by Iron Oxide and Silica Doped 3D Printed Tricalcium Phosphate Scaffolds. Ann. Biomed. Eng. 2018;46:1241–1253. doi: 10.1007/s10439-018-2040-8. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
45. Li Y., Wu Q., Tang G., Li H., Shang L.Y. Printing Preparation and Performance Characteristics of Magnesium Bone Tissue Engineering Scaffold. Res. Tissue Eng. China. 2018;22:827–832. [Google Scholar]
46. Lai Y., Li Y., Cao H., Long J., Wang X., Li L., Li C. , Jia Q., Teng B., Tang T., et al. Osteogenic Magnesium Incorporated into PLGA/TCP Porous Scaffold by 3D Printing for Repairing Challenging Bone Defect. Biomaterials. 2019;197:207–219. doi: 10.1016/j.biomaterials.2019.01.013. [PubMed] [CrossRef] [Google Scholar]
, Jia Q., Teng B., Tang T., et al. Osteogenic Magnesium Incorporated into PLGA/TCP Porous Scaffold by 3D Printing for Repairing Challenging Bone Defect. Biomaterials. 2019;197:207–219. doi: 10.1016/j.biomaterials.2019.01.013. [PubMed] [CrossRef] [Google Scholar]
47. Zhang J.H., Zhao S.H., Zhu M., Zhu Y.F., Zhang Y.D., Liu Z.T., Zhang C.Q. 3D Printed Magnetic Fe3O4/MBG/PCL Composite Scaffolds with Multifunctionality of Bone Regeneration, Local Anticancer Drug Delivery and Hyperthermia. J. Mater. Chem. B. 2014;2:7583–7595. doi: 10.1039/C4TB01063A. [PubMed] [CrossRef] [Google Scholar]
48. Li Y., Jahr H., Lietaert K., Pavanram P., Yilmaz A., Fockaert L.I., Leeflang M.A., Pouran B., Garcia Y.G., Weinans H., et al. Additively Manufactured Biodegradable Porous Iron. Acta Biomater. 2018;77:380–393. doi: 10.1016/j.actbio.2018.07.011. [PubMed] [CrossRef] [Google Scholar]
49. Jauer L., Kimm M., Meiners W., Batista R.S., Labude N., Bienert M., Schleifenbaum J. Additive Manufacturing of Magnesium Alloys; Proceedings of the 9th Symposium on Biodegradable Metals; Bertinoro, Italy. 27 August–1 September 2017. [Google Scholar]
27 August–1 September 2017. [Google Scholar]
50. Li Y., Zhou J., Pavanram P., Leeflang M.A., Fockaert L.I., Pouran B., Tümer N., Schröder K.-U., Mol J.M.C., Weinans H., et al. Additively Manufactured Biodegradable Porous Magnesium. Acta Biomater. 2018;67:378. doi: 10.1016/j.actbio.2017.12.008. [PubMed] [CrossRef] [Google Scholar]
51. Li Y., Jahr H., Zhang X.Y., Leeflang M.A., Li W., Pouran B., Tichelaar F.D., Weinans H., Zhou J., Zadpoor A.A. Biodegradation affected fatigue behavior of additively manufactured porous magnesium. Addit. Manuf. 2019;28:299. doi: 10.1016/j.addma.2019.05.013. [CrossRef] [Google Scholar]
52. Pei X., Ma L., Zhang B., Sun J., Sun Y., Fan Y., Gou Z., Zhou C., Zhang X. Creating Hierarchical Porosity Hydroxyapatite Scaffolds with Osteo Induction by Three Di Mensional Printing and Microwave Sintering. Biofabrication. 2017;9:045008. doi: 10.1088/1758-5090/aa90ed. [PubMed] [CrossRef] [Google Scholar]
53. Chen Z., Zhang X., Yang Y., Zhou K., Wragg N. , Liu Y., Lewis M., Liu C. Fabrication and Characterization of 3D Complex Hydroxyapatite Scaffolds with Hierarchical Porosity of Different Features for Optimal Bioactive Performance. Ceram. Int. 2017;43:336–344. doi: 10.1016/j.ceramint.2016.09.160. [CrossRef] [Google Scholar]
, Liu Y., Lewis M., Liu C. Fabrication and Characterization of 3D Complex Hydroxyapatite Scaffolds with Hierarchical Porosity of Different Features for Optimal Bioactive Performance. Ceram. Int. 2017;43:336–344. doi: 10.1016/j.ceramint.2016.09.160. [CrossRef] [Google Scholar]
54. Ren X., Tuo Q., Tian K., Huang G., Li J., Xu T., Lv X., Wu J., Chen Z., Weng J., et al. Enhancement of Osteogenesis Using a Novel Porous Hydro Xyapatite Scaffold In Vivo and Vitro. Ceram. Int. 2018;44:21656–21665. doi: 10.1016/j.ceramint.2018.08.249. [CrossRef] [Google Scholar]
55. Calabrese G., Giuffrida R., Forte S., Fabbi C., Figallo E., Salvatorelli L., Memeo L., Parenti R., Gulisano M., Gulino R. Human Adipose Derived Mesenchymal Stem-cells Seeded into A Collagen Hydroxyapatite Scaffold Promote Bone Augmentation after Implantation in the Mouse. Sci. Rep. 2017;7:7110. doi: 10.1038/s41598-017-07672-0. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
56. Wang R.H., Cheng L.G., Chen K.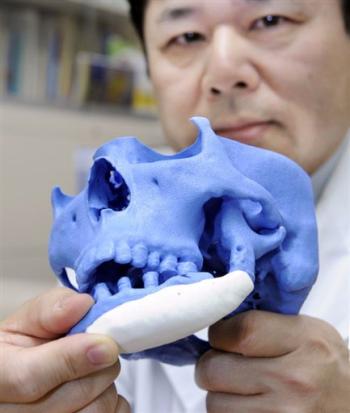 Application and Value of Polymer Materials in 3D Printing Biological Bones and Scaffolds. Res. Tissue Eng. China. 2022;26:610–616. [Google Scholar]
Application and Value of Polymer Materials in 3D Printing Biological Bones and Scaffolds. Res. Tissue Eng. China. 2022;26:610–616. [Google Scholar]
57. Zhang H.F., Mao X.Y., Zhao D.Y., Jiang W.B., Du Z., Li Q., Jiang C., Han D. Experimental Study on 3D Printing PLA-HA Composite Material to Construct Tissue Engineering Bone. Int. J. Orthop. 2016;37:57–63. [Google Scholar]
58. Cavo M., Scaglione S. Scaffold Microstructure Effects on Functional and Mechanical Performance: Integration of Theoretical and Experimental Approaches for Bone Tissue Engineering Applications. Mater. Sci. Eng. C. 2016;68:872–879. doi: 10.1016/j.msec.2016.07.041. [PubMed] [CrossRef] [Google Scholar]
59. Zhang S., Ma B., Liu F., Duan J., Wang S., Qiu J., Li D., Sang Y., Liu C., Liu D., et al. Polylactic Acid Nanopillar Array-Driven Osteogenic Differentiation of Human Adipose-Derived Stem Cells Determined by Pillar Diameter. Nano Lett. 2018;18:2243–2253. doi: 10.1021/acs.nanolett.7b04747. [PubMed] [CrossRef] [Google Scholar]
60. Zhang G.Q., Wang Y., Zhou D., Jun J., Jin X., Jiang T. Preparation and Histocompatibility of Polycaprolactone/Poly-triethyene Carbonate Electrospinning Scaffold Material. J. Shanxi Med. Univ. 2018;49:251–257. [Google Scholar]
Zhang G.Q., Wang Y., Zhou D., Jun J., Jin X., Jiang T. Preparation and Histocompatibility of Polycaprolactone/Poly-triethyene Carbonate Electrospinning Scaffold Material. J. Shanxi Med. Univ. 2018;49:251–257. [Google Scholar]
61. Yu C., Liu K., Hou C.J., He T., Deng W.S., Sun P. Biocompatibility of 3D printed nano-hydroxyapatite/polycaprolactone scaffolds. J. Med. Coll. Qingdao Univ. 2017;53:1–4. [Google Scholar]
62. Seidenstuecker M., Kerr L., Bernstein A., Mayr H., Suedkamp N.P., Gadow R., Krieg P., Hernandez L.S., Thomann R., Syrowatka F., et al. 3D Powder Printed Bio-glass and β-Tricalcium Phosphate Bone Scaffolds. Materials. 2018;11:13. doi: 10.3390/ma11010013. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
63. Li X., Wang Y., Wang Z., Qi Y., Li L., Zhang P., Chen X., Huang Y. Composite PLA/PEG/n HA/Dexamethasone scaffold prepared by 3d printing for bone regeneration. Macromol. Biosci. 2018;18:1800068. doi: 10.1002/mabi.201800068. [PubMed] [CrossRef] [Google Scholar]
64. Kim C.G., Han K.S., Lee S., Kim M.C., Kim S.Y., Nah J. Fabrication of Biocompatible Polycaprolactone–Hydroxyapatite Composite Filaments for the FDM 3D Printing of Bone Scaffolds. Appl. Sci. 2021;11:6351. doi: 10.3390/app11146351. [CrossRef] [Google Scholar]
Kim C.G., Han K.S., Lee S., Kim M.C., Kim S.Y., Nah J. Fabrication of Biocompatible Polycaprolactone–Hydroxyapatite Composite Filaments for the FDM 3D Printing of Bone Scaffolds. Appl. Sci. 2021;11:6351. doi: 10.3390/app11146351. [CrossRef] [Google Scholar]
65. Kawai T., Shanjani Y., Fazeli S., Behn A.W., Okuzu Y., Goodman S.B., Yang Y.P. Customized, Degradable, Functionally Graded Scaffold for Potential Treatment of Early-Stage Osteonecrosis of the Femoral Head. J. Orthop. Res. 2018;36:1002–1011. doi: 10.1002/jor.23673. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
66. Xu H., Han D., Dong J.-S., Shen G.-X., Chai G., Yu Z.-Y., Lang W.-J., Ai S.-T. Rapid Prototyped PGA/PLA Scaffolds in The Reconstruction of Mandibular Condyle Bone Defects. Int. J. Med. Robot. 2010;6:66–72. doi: 10.1002/rcs.290. [PubMed] [CrossRef] [Google Scholar]
67. Zhang B., Shen S., Xian H., Dai Y.J., Guo W.M., Li X., Zhang X.L., Wang Z.Y., Li H.J., Peng L.Q., et al. Study on PLGA/ Acellular Cartilage Extracellular Matrix Scaffold Material Prepared by 3D Printing and its Physicochemical Properties. Chin. J. Repair Reconstr. Surg. 2019;33:1011–1018. [PMC free article] [PubMed] [Google Scholar]
Chin. J. Repair Reconstr. Surg. 2019;33:1011–1018. [PMC free article] [PubMed] [Google Scholar]
68. Gu X.M. Ph.D. Thesis. Jilin University; Changchun, China: 2021. Preparation and Osteogenesis of 3D Printed Porous Amorphous Poly (Aryl Ether Ketone) Composite Scaffold. [Google Scholar]
69. Li S. Surface Porous Poly-Ether-Ether-Ketone Based on Three-Dimensional Printing for Load Bearing Orthopedic Implant. J. Mech. Behav. Biomed. Mater. 2021;120:104561. doi: 10.1016/j.jmbbm.2021.104561. [PubMed] [CrossRef] [Google Scholar]
70. Polley C., Distler T., Detsch R., Lund H., Springer A., Boccaccini A.R., Seitz H. 3D Printing of Piezoelectric Barium Titanate-Hydroxyapatite Scaffolds with Interconnected Porosity for Bone Tissue Engineering. Materials. 2020;13:1773. doi: 10.3390/ma13071773. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
71. Zhao B.H., Li Y., Li W.J. Study on The Mechanical Properties of Poly-Ether-Ether-Ketone, an Orthopedic Implant Material Prepared by 3D Printing Technology and the Influence of Amino Modification on the Osteogenic Properties of the Material. Inf. Chin. Med. Devices. 2021;27:10–12. [Google Scholar]
Inf. Chin. Med. Devices. 2021;27:10–12. [Google Scholar]
72. Jia W.Y., Cui D., Liu Y., Ji X., Sun M.L., Cheng Z.Q., Luo Y.G., Liu G.M. Polyether-Ether-Ketone/ Poly (Methyl Methacrylate)/Carbon Fiber Ternary Composites Prepared by Electrospinning and Hot Pressing for Bone Implant Applications. Mater. Des. 2021;209:109893. doi: 10.1016/j.matdes.2021.109893. [CrossRef] [Google Scholar]
73. Soundarya S.P., Menon A.H., Chandran S.V., Selvamurugan N. Bone Tissue Engineering: Scaffold Preparation Using Chitosan and other Biomaterials with Different Design and Fabrication Techniques. Int. J. Biol. Macromol. 2018;119:1228–1239. doi: 10.1016/j.ijbiomac.2018.08.056. [PubMed] [CrossRef] [Google Scholar]
74. Saravanan S., Leena R.S., Selvamurugan N. Chitosan Based Bio-Composite Scaffolds for Bone Tissue Engineering. Int. J. Biol. Macromol. 2016;93:1354–1365. doi: 10.1016/j.ijbiomac.2016.01.112. [PubMed] [CrossRef] [Google Scholar]
75. Lavanya K., Chandran S.V., Balagangadharan K. , Selvamurugan N. Temperature-And Ph-Responsive Chitosan-Based Injectable Hydrogels for Bone Tissue Engineering. Mater. Sci. Eng. C. 2020;111:110862. doi: 10.1016/j.msec.2020.110862. [PubMed] [CrossRef] [Google Scholar]
, Selvamurugan N. Temperature-And Ph-Responsive Chitosan-Based Injectable Hydrogels for Bone Tissue Engineering. Mater. Sci. Eng. C. 2020;111:110862. doi: 10.1016/j.msec.2020.110862. [PubMed] [CrossRef] [Google Scholar]
76. Yu H.Y., Ma D.D., Wu B.L. Adhesion and Proliferation Effect of 3D Printed Gelatin Sodium Alginate Gel Scaffold on Human Dental Pulp Cells. J. South. Med. Univ. 2017;37:668–672. [PMC free article] [PubMed] [Google Scholar]
77. Yuan Q.X., Gao L.L., Li R.X., Liu Y.J., Lin X.L., Zhang X.Z. 3D Printing Silk Fibroin-Type ⅱ Collagen Cartilage Scaffold. J. Shandong Univ. (Sci. Ed.) 2018;53:82–87. [Google Scholar]
78. Lu Y.N., Zhang X.Z., Lin B.B., Xu G., Chen J.D., Chen S.Y. Repair of Rat Skull Defect with Naringin-Chitosan/Hydroxyapatite Composite Scaffold. Chin. Tissue Eng. Res. 2022;26:4441–4445. [Google Scholar]
79. Luo W.B. Ph.D. Thesis. Jilin University; Changchun, China: 2020. Cellulose Nanofiber Modified Sodium Alginate Gelatin Bio-Ink Is Used for Biological 3D Printing of Cell Components at Ligament Bone Interface. [Google Scholar]
[Google Scholar]
80. Liu J. Ph.D. Thesis. Huazhong University of Science and Technology; Wuhan, China: 2019. Research on Cu-Containing Mesoporous Bioactive Glass/Sodium Alginate Composite Bone Tissue Engineering Scaffold Based on Ultra-Low Temperature Extrusion Deposition Technology. [Google Scholar]
81. Sing S.L., Wang S., Agarwala S., Florencia E.W., Thi M.H.H., Wai Y.Y. Fabrication of Titanium Based Biphasic Scaffold Using Selective Laser Melting and Collagen Immersion. Int. J. Bioprinting. 2017;3:65–71. doi: 10.18063/IJB.2017.01.007. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
82. He J., Li Z.L., Xie Z.G. Research Progress of Osteo-inductive Properties of Bone Substitute Materials. Oral Dis. Prev. 2018;26:124–127. [Google Scholar]
83. Zhou H.B., Yu Z.W., Liu J.G. The Molecular Mechanism of Medical Metal Implants to Promote Bone Regeneration. China Tissue Eng. Res. 2022;26:1588–1596. [Google Scholar]
84. Chocholata P., Kulda V., Babuska V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials. 2019;12:568. doi: 10.3390/ma12040568. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Materials. 2019;12:568. doi: 10.3390/ma12040568. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
85. Zhou X., Feng Y., Zhang J., Shi Y., Wang L. Recent Advances in Additive Manufacturing Technology for Bone Tissue Engineering Scaffolds. Int. J. Adv. Manuf. Technol. 2020;108:3591–3606. doi: 10.1007/s00170-020-05444-1. [CrossRef] [Google Scholar]
86. Shen C., Witek L., Flores R.L., Tovar N., Torroni A., Coelho P.G., Kasper F.K., Wong M., Young S. Three-Dimensional Printing for Craniofacial Bone Tissue Engineering. Tissue Eng. Part A. 2020;26:23–24. doi: 10.1089/ten.tea.2020.0186. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
87. Bagde A.D., Kuthe A.M., Quazi S., Gupta V., Jaiswal S., Jyothilal S., Lande N., Nagdeve S. State of The Art Technology for Bone Tissue Engineering and Drug Delivery. IRBM. 2019;40:133–144. doi: 10.1016/j.irbm.2019.03.001. [CrossRef] [Google Scholar]
88. Tan C.L., Li S., Khamis E., Parastoo J., Zhou K.S., Ma W.Y., Moataz M. , Attallah M.A. Laser Powder Bed Fusion of Ti-Rich Tini Lattice Structures: Process Optimisation, Geometrical Integrity, and Phase Transformations. Int. J. Mach. Tools Manuf. 2019;141:19–29. doi: 10.1016/j.ijmachtools.2019.04.002. [CrossRef] [Google Scholar]
, Attallah M.A. Laser Powder Bed Fusion of Ti-Rich Tini Lattice Structures: Process Optimisation, Geometrical Integrity, and Phase Transformations. Int. J. Mach. Tools Manuf. 2019;141:19–29. doi: 10.1016/j.ijmachtools.2019.04.002. [CrossRef] [Google Scholar]
89. Li J., Chen M., Fan X., Zhou H. Recent Advances in Bioprinting Techniques: Approaches, Applications and Future Prospects. J. Transl. Med. 2016;14:271. doi: 10.1186/s12967-016-1028-0. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
90. Akesh B., Kakarla I.K., Turek I., Kong C., Helen I. Printable Gelatin, Alginate and Boron Nitride Nanotubes Hydrogel-Based Ink For 3D Bioprinting and Tissue Engineering Applications. Mater. Des. 2022;213:110362. [Google Scholar]
91. Ceng H., Wang M., Chen D., Shi B., Bai Y. Process and Biological Properties of Biphasic Calcium Phosphate Bone Tissue Engineering Scaffold Made by Selective Laser Sintering Technology. Stomato-Log. Res. 2018;34:165–168. [Google Scholar]
92. Ruben W., Johan V.D.S., Saber A.Y., Jan V.H., Jean P.K., Amir A.Z., Harrie W., Michiel M., Jan S. Additively Manufactured Porous Tantalum Implants. Acta Biomater. 2015;14:217–225. [PubMed] [Google Scholar]
Ruben W., Johan V.D.S., Saber A.Y., Jan V.H., Jean P.K., Amir A.Z., Harrie W., Michiel M., Jan S. Additively Manufactured Porous Tantalum Implants. Acta Biomater. 2015;14:217–225. [PubMed] [Google Scholar]
93. Guo L.Y. Study on The Construction of HA/PCL Scaffold for Bone Tissue Engineering by Selective Laser Sintering Technology. Chin. J. Dent. Mater. Devices. 2015;24:64–68. [Google Scholar]
94. Song H.S., Li W., Song P.H., Su Q.Y., Wei Q.S., Shi Y.S., Liu K., Liu W.G. Selective Laser Sintering of Aliphatic-Polycarbonate/Hydroxyapatite Composite Scaffolds for Medical Applications. Int. J. Adv. Manuf. Technol. 2015;81:15–25. [Google Scholar]
95. Jun S., Jun Z., Qi F.W., Wang G.Y., Liu Z., Yang Y.W., Peng S.P. n-MgO Incorporated PLLA Bone Scaffolds: Enhanced Crystallinity and Neutralized Acidic Products. Mater. Des. 2019;174:107801. [Google Scholar]
96. Dong Q.S., Zhang M., Zhou X., Shao Y., Li J., Wang L., Chu C., Xue F., Yao Q., Bai J. 3D Printed Mg Incorporated PCL Based Scaffolds: A Promising Approach for Bone Healing. Mater. Sci. Eng. C. 2021;129:112372. doi: 10.1016/j.msec.2021.112372. [PubMed] [CrossRef] [Google Scholar]
Mater. Sci. Eng. C. 2021;129:112372. doi: 10.1016/j.msec.2021.112372. [PubMed] [CrossRef] [Google Scholar]
97. Bulina N.V., Baev S.G., Makarova S.V., Vorobyev A.M., Titkov A.I., Bessmeltsev V.P., Lyakhov N.Z. Selective Laser Melting of Hydroxyapatite: Perspectives for 3D Printing of Bioresorbable Ceramic Implants. Materials. 2021;14:18. doi: 10.3390/ma14185425. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
98. He H.Y., Zhang J.Y., Mi X., Hu Y., Gu X.Y. Rapid Prototyping for Tissue-Engineered Bone Scaffold by 3D Printing and Biocompatibility Study. Int. J. Clin. Exp. Med. 2015;8:11777–11785. [PMC free article] [PubMed] [Google Scholar]
99. Zhang H.F., Du Z.J., Mao X., Dan Y.Z., Chao D., Wen B.J., Dong H. Experimental Study on Tissue Engineering Bone Constructed by 3D Printing PLA-HA Composite Material. Int. J. Orthop. 2016;37:57–63. [Google Scholar]
100. Jiao X., Liu Z., Li X., Jian F., Wei M.Y. Study On Fabrication Of HA/PCL Composite Tissue Engineering Scaffolds by 3D Printing. J. Beijing Univ. Chem. Technol. 2018;48:14952–14963. [Google Scholar]
J. Beijing Univ. Chem. Technol. 2018;48:14952–14963. [Google Scholar]
101. Xu N., Ye X., Wei D., Zhong J., Chen Y., Xu G., He D. 3D Artificial Bones for Bone Repair Prepared by Computed Tomography Guided Fused Deposition Modeling for Bone Repair. ACS Appl. Mater. Interfaces. 2014;6:14952–14963. doi: 10.1021/am502716t. [PubMed] [CrossRef] [Google Scholar]
102. Senem B., Tugba E.T., Arda B., Ezgi I.B., Gamze T.K., Deniz Y., Tahsin B., Engin C., Cagri Y., Ergin T. 3D Printed Poly(Ε-Caprolactone) Scaffolds Modified with Hydroxyapatite and Poly (Propylene Fumarate) and their Effects on the Healing of Rabbit Femur Defects. Biomater. Sci. 2017;5:2144–2158. [PubMed] [Google Scholar]
103. Lian Q., Wu X., Li D., He X., Meng J., Liu X., Jin Z. Accurate Printing of a Zirconia Molar Crown Bridge Using Three Part Auxiliary Supports and Ceramic Mask Projection Stereolithography. Ceram. Int. 2019;45:15. doi: 10.1016/j.ceramint.2019.06.111. [CrossRef] [Google Scholar]
104. Ding R. , Wu Z.H., Qiu G.X., Wu G., Wang H., Su X.L., Yoon P., Ma S., Qi B. Bone Tissue Engineering Observation of Porous Titanium Alloy Scaffold by Selective Laser Sintering Technology. Chin. J. Med. 2014;94:1499–1502. [PubMed] [Google Scholar]
, Wu Z.H., Qiu G.X., Wu G., Wang H., Su X.L., Yoon P., Ma S., Qi B. Bone Tissue Engineering Observation of Porous Titanium Alloy Scaffold by Selective Laser Sintering Technology. Chin. J. Med. 2014;94:1499–1502. [PubMed] [Google Scholar]
105. Chen Y.W., Shie M.-Y., Chang W.C., Shen Y.F. Approximate Optimization Study of Light Curing Waterborne Polyurethane Materials for the Construction of 3D Printed Cyto-compatible Cartilage Scaffolds. Materials. 2021;14:6804. doi: 10.3390/ma14226804. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
106. Elomaa L., Teixeira S., Hakala R., Korhonen H., Grijpma D.W., Seppälä J.V. Preparation of poly (ε-caprolactone)-based tissue engineering scaffolds by stereolithography. Acta Biomater. 2011;7:3850–3856. doi: 10.1016/j.actbio.2011.06.039. [PubMed] [CrossRef] [Google Scholar]
107. Jia B.Z., Ma Z., Hong Q.C., Yun L. Study on Preparation and Properties of 45S5 Bioactive Bone Tissue Scaffold by 3D Printing. Beijing Biomed. Eng. 2019;37:6. [Google Scholar]
[Google Scholar]
108. Tesavibul P., Felzmann R., Gruber S. Processing of 45S5 Bio-glass by Lithography Based Additive Manufacturing. Mater. Lett. 2012;74:81–84. doi: 10.1016/j.matlet.2012.01.019. [CrossRef] [Google Scholar]
109. Senatov F.S., Niaza K.V., Zadorozhnyy Y.M., Maksimkin A.V., Kaloshkin S.D., Estrin Y.Z. Mechanical Properties and Shape Memory Effect of 3D-Printed PLA-Based Porous Scaffolds. J. Mech. Behav. Biomed. Mater. 2016;57:139–148. doi: 10.1016/j.jmbbm.2015.11.036. [PubMed] [CrossRef] [Google Scholar]
110. Guo L., Jian B.Y., Yan X., Wang Z., Huang Y.L. Construction of HA/PCL Bone Tissue Engineering Scaffold by Selective Laser Sintering. J. Dental Mater. Instrum. 2020;24:64–68. [Google Scholar]
111. Jin G.H., Xiao F.S., Yan X., Zhang Y.T., Li C., Yang Q.Q., Wang Z.K., Xie Y. Construction of Nano-Hydroxyapatite and Polycaprolactone Composite Artificial Bone Scaffolds by Selective Laser Sintering. Acad. J. Second Mil Univ. 2015;36:1289–1294. doi: 10. 3724/SP.J.1008.2015.01289. [CrossRef] [Google Scholar]
3724/SP.J.1008.2015.01289. [CrossRef] [Google Scholar]
112. Yoo J.J., Kim H.J., Seo S.M. Preparation of a Hemi porous Hydroxyapatite Scaffold and Evaluation as a Cell Mediated Bone Substitute. Ceram Int. 2014;40:3079–3087. [Google Scholar]
113. Yin B., Xue B.J., Wu Z.H., Ma J., Wang K. A Novel Hybrid 3D Printed Titanium Scaffold for Osteogenesis in A Rabbit Calvarial Defect Model. Am. J. Transl. Res. 2018;10:474–482. [PMC free article] [PubMed] [Google Scholar]
114. Poldervaart M.T., Wang H., van der Stok J., Weinans H., Leeuwenburgh S.C.G., Öner F., Dhert W.J.A., Alblas J. Sustained Release of BMP-2 In Bio-printed Alginate for Osteogenicity in Mice and Rats. PLoS ONE. 2013;8:72610 [PMC free article] [PubMed] [Google Scholar]
115. Ahn C.B., Kim Y., Park S.J., Hwang Y., Lee J.W. Development of Arginine Glycine Aspartate Immobilized 3D Printed Poly (Propylene Fumarate) Scaffolds for Cartilage Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2017;29:1–27. doi: 10.1080/09205063. 2017.1383020. [PubMed] [CrossRef] [Google Scholar]
2017.1383020. [PubMed] [CrossRef] [Google Scholar]
116. Kang H.W., Lee S.J., Ko I.K., Kengla C., Yoo J.J., Atala A. A 3D Bioprinting System to Produce Human-Scale Tissue Constructs with Structural Integrity. Nat. Biotechnol. 2016;34:312. doi: 10.1038/nbt.3413. [PubMed] [CrossRef] [Google Scholar]
117. Yang Z., Ran Q., Tong C., Zhang K.B., Gui J.C. 3D Bioprinting Modified Autologous Matrix Induced Chondrogenesis (AMIC) Technique for Repair of Cartilage Defects. Mater. Des. 2021;203:109621. [Google Scholar]
118. Guo T., Maeesha N., Hannah B., Baker E.T., Sean J.M., Tang Q.G., Julia P.R., Max J.L., Chen Y., Jonathan D., et al. 3D Printed Biofunctionalized Scaffolds for Microfracture Repair of Cartilage Defects. Biomaterials. 2018;185:219–231. doi: 10.1016/j.biomaterials.2018.09.022. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
119. Sa L., Lina M., Bi G.Y., Chen S.G., Yan D.W., Yang J.H., Jia Y.G., Li R. DLP 3D Printing Porous Β-Tricalcium Phosphate Scaffold by the Use of Acrylate/Ceramic Composite Slurry. Ceram. Int. 2021;47:21108–21116. [Google Scholar]
Ceram. Int. 2021;47:21108–21116. [Google Scholar]
120. Yong S.C., Yang S.Y., Choi E.C., Kim K.H., Gwak S.J. Fabrication of A Porous Hydroxyapatite Scaffold with Enhanced Human Osteoblast-Like Cell Response Via Digital Light Processing System and Biomimetic Mineralization. Ceram. Int. 2021;47:35134–35143. [Google Scholar]
121. Lim H.K., Ryu M., Woo S.H., Song I.S., Choi Y.J., Lee U.L. Bone Conduction Capacity of Highly Porous 3D-Printed Titanium Scaffolds Based on Different Pore Designs. Materials. 2021;14:3892. doi: 10.3390/ma14143892. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
122. Wie D.J., Wolf A., Bader R. Numerical Optimization of Open-Porous Bone Scaffold Structures to Match the Elastic Properties of Human Cortical Bone. J. Mech. Behav. Biomed. Mater. 2014;37:56–68. [PubMed] [Google Scholar]
123. Liu Z.B., Liang H.X., Shi T.S., Xie D.Q., Chen R.Y., Han X., Shen L.D., Wang C.J., Tian Z.J. Additive Manufacturing of Hydroxyapatite Bone Scaffolds via Digital Light Processing and in Vitro Compatibility. Ceram. Int. 2019;45:11079–11086. doi: 10.1016/j.ceramint.2019.02.195. [CrossRef] [Google Scholar]
Ceram. Int. 2019;45:11079–11086. doi: 10.1016/j.ceramint.2019.02.195. [CrossRef] [Google Scholar]
124. Zhou X. Master’s Thesis. Qilu University of Technology; Jinan, China: 2021. 3D Printing and Performance Study of Meteorite Bone Tissue Engineering Scaffold. [Google Scholar]
125. Safonov A., Maltsev E., Chugunov S., Tikhonov A., Konev S., Evlashin S., Popov D., Pasko A., Akhatov I. Design and Fabrication of Complex-Shaped Ceramic Bone Implants via 3D Printing Based on Laser Stereolithography. Appl. Sci. 2020;10:7138. doi: 10.3390/app10207138. [CrossRef] [Google Scholar]
126. Ma J.Q., Lin L.L., Zuo Y., Zou Q., Xin R., Li J.D., Li Y.B. Modification of 3D Printed PCL Scaffolds by PVAC and HA to Enhance Cytocompatibility and Osteogenesis. RSC Adv. 2019;9:5338–5353. doi: 10.1039/C8RA06652C. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
127. Liu J., Zou Q., Wang C.X., Lin M.Y., Li Y.F., Zhang R., Li Y.B. Electrospinning and 3D Printed Hybrid Bi-Layer Scaffold for Guided Bone Regeneration.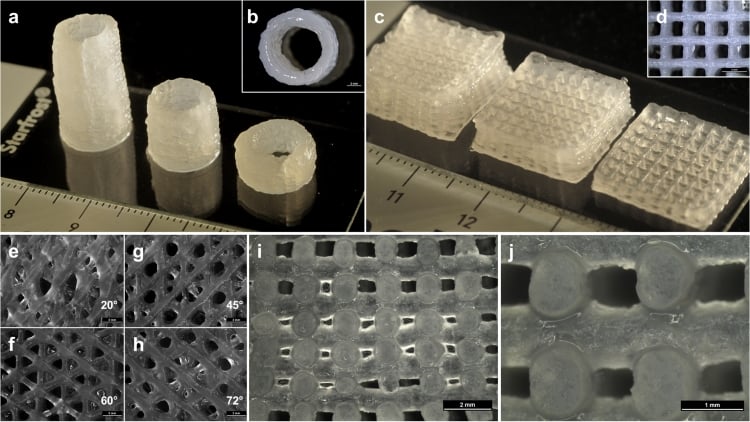 Mater. Des. 2021;210:110147. doi: 10.1016/j.matdes.2021.110047. [CrossRef] [Google Scholar]
Mater. Des. 2021;210:110147. doi: 10.1016/j.matdes.2021.110047. [CrossRef] [Google Scholar]
128. Wang Y.J., Sajad A., Michael T., Daminao P. Hip Implant Design with Three-Dimensional Porous Architecture of Optimized Graded Density. J. Mech. Des. 2018;140:111406. doi: 10.1115/1.4041208. [CrossRef] [Google Scholar]
129. Park D.W., Lim A., Park J.W., Lim K.M., Kang H.G. Biomechanical Evaluation of a New Fixation Type in 3D Printed Periacetabular Implants using a Finite Element Simulation. Appl. Sci. 2019;9:820. doi: 10.3390/app9050820. [CrossRef] [Google Scholar]
130. Rick F., Dong J.F. Surface Modification of Bone Tissue Engineering Implants. Res. Tissue Eng. China. 2020;22:3573–3578. [Google Scholar]
131. Weingartner L., Latorre S.H., Velten D., Bernstein A., Schmal H., Seidenstuecker M. The Effect of Collagen-I Coatings of 3D Printed PCL Scaffolds for Bone Replacement on Three Different Cell Types. Appl. Sci. 2021;11:11063. doi: 10.3390/app112211063. [CrossRef] [Google Scholar]
132. Sun K.Y., Xu M.G., Zhou Y.Y. Study on β-TCP Bone Tissue Engineering Scaffold Coated with Type I Collagen Based On 3D Printing. Chin. J. Biomed. Eng. 2018;37:335–343. [Google Scholar]
Sun K.Y., Xu M.G., Zhou Y.Y. Study on β-TCP Bone Tissue Engineering Scaffold Coated with Type I Collagen Based On 3D Printing. Chin. J. Biomed. Eng. 2018;37:335–343. [Google Scholar]
133. Poh H.D.W., Stevens M.M. Fabrication and in Vitro Characterization of Bioactive Glass Composite Scaffolds for Bone Regeneration. Biofabrication. 2014;6:029501. doi: 10.1088/1758-5082/6/2/029501. [PubMed] [CrossRef] [Google Scholar]
134. Zhang Y., Xia I., Zhai D., Shi M., Luo Y., Feng C., Wu C. Mesoporous Bioactive Glass Nanolayer-Functionalized 3D Printed Scaffolds for Accelerating Osteogenesis and Angiogenesis. Nanoscale. 2015;7:19207–19221. doi: 10.1039/C5NR05421D. [PubMed] [CrossRef] [Google Scholar]
135. Remy M.T., Akkouch A., He L., Eliason S., Sweat M.E., Krongbaramee T., Fei F., Qian F., Amendt B.A., Song X., et al. Rat Calvarial Bone Regeneration by 3D-Printed β-Tricalcium Phosphate Incorporating MicroRNA-200c. ACS Biomater. Sci. Eng. 2021;7:4521–4534. doi: 10.1021/acsbiomaterials. 0c01756. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
0c01756. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
136. Nasrin F., Ehsan A., Shiva I., Abdolreza A., Ehsan S. 3D Printed PCL Scaffolds Coated with Nano Bio-ceramics Enhance Osteogenic Differentiation of Stem Cells. ACS Omega. 2021;6:35284–35296. [PMC free article] [PubMed] [Google Scholar]
137. Fu Z.Q., Ouyang L.L., Xu R.Z., Yang Y., Sun W. Responsive Biomaterials for 3D Bioprinting: A Review. Mater. Today. 2022 doi: 10.1016/j.mattod.2022.01.001. in press . [CrossRef] [Google Scholar]
138. Kirillova A., Maxson R., Stoychev G., Ionov L. 4D Bio-fabrication Using Shape-Morphing Hydrogels. Adv. Mater. 2017;29:1703443. doi: 10.1002/adma.201703443. [PubMed] [CrossRef] [Google Scholar]
139. Wu S.D., Hsu S.H. 4D Bio-printable Self-healing Hydrogel with Shape Memory and Cryopreserving Properties. Biofabrication. 2021;13:045029. doi: 10.1088/1758-5090/ac2789. [PubMed] [CrossRef] [Google Scholar]
3D Printing of Personalized Artificial Bone Scaffolds
1. Bhumiratana S, Vunjak-Novakovic G. Concise review: personalized human bone grafts for reconstructing head and face. Stem Cells Transl Med 2012;1:64–69 [PMC free article] [PubMed] [Google Scholar]
Bhumiratana S, Vunjak-Novakovic G. Concise review: personalized human bone grafts for reconstructing head and face. Stem Cells Transl Med 2012;1:64–69 [PMC free article] [PubMed] [Google Scholar]
2. De Long WG, Jr., Einhorn TA, Koval K, et al.. Bone grafts and bone graft substitutes in orthopaedic trauma surgery: a critical analysis. J Bone Joint Surg 2007;89:649–658 [PubMed] [Google Scholar]
3. Kurien T, Pearson RG, Scammell BE. Bone graft substitutes currently available in orthopaedic practice: the evidence for their use. Bone Joint J 2013;95-B:583–597 [PubMed] [Google Scholar]
4. Zhang J, Liu W, Schnitzler V, et al.. Calcium phosphate cements for bone substitution: chemistry, handling and mechanical properties. Acta Biomater 2014;10:1035–1049 [PubMed] [Google Scholar]
5. Bohner M, Gbureck U, Barralet J. Technological issues for the development of more efficient calcium phosphate bone cements: a critical assessment. Biomaterials 2005;26:6423–6429 [PubMed] [Google Scholar]
6. Murtha PE, Hafez MA, Jaramaz B, DiGioia AM., 3rd Variations in acetabular anatomy with reference to total hip replacement. J Bone Joint Surg Br 2008;90:308–313 [PubMed] [Google Scholar]
Murtha PE, Hafez MA, Jaramaz B, DiGioia AM., 3rd Variations in acetabular anatomy with reference to total hip replacement. J Bone Joint Surg Br 2008;90:308–313 [PubMed] [Google Scholar]
7. McNiesh LM, Callaghan JJ. CT arthrography of the shoulder: variations of the glenoid labrum. AJR Am J Roentgenol 1987;149:963–966 [PubMed] [Google Scholar]
8. Reichert JC, Wullschleger ME, Cipitria A, et al.. Custom-made composite scaffolds for segmental defect repair in long bones. Int Orthop 2011;35:1229–1236 [PMC free article] [PubMed] [Google Scholar]
9. Mota C, Puppi D, Chiellini F, Chiellini E. Additive manufacturing techniques for the production of tissue engineering constructs. J Tissue Eng Regen Med 2015;9:174–190 [PubMed] [Google Scholar]
10. Sun W, Darling A, Starly B, Nam J. Computer-aided tissue engineering: overview, scope and challenges. Biotechnol Appl Biochem 2004;39:29–47 [PubMed] [Google Scholar]
11. Ciocca L, De Crescenzio F, Fantini M, Scotti R. CAD/CAM and rapid prototyped scaffold construction for bone regenerative medicine and surgical transfer of virtual planning: a pilot study. Comput Med Imaging Graph 2009;33:58–62 [PubMed] [Google Scholar]
Comput Med Imaging Graph 2009;33:58–62 [PubMed] [Google Scholar]
12. Yang S, Leong K, Du Z, Chua C. The design of scaffolds for use in tissue engineering. Part II. Rapid prototyping techniques. Tissue Eng 2002;8:1–11 [PubMed] [Google Scholar]
13. Seitz H, Rieder W, Irsen S, et al.. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater 2005;74:782–788 [PubMed] [Google Scholar]
14. Leukers B, Gülkan H, Irsen SH, et al.. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J Mater Sci Mater Med 2005;16:1121–1124 [PubMed] [Google Scholar]
15. Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today 2013;16:496–504 [Google Scholar]
16. Hutmacher DW, Sittinger M, Risbud MV. Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol 2004;22:354–362 [PubMed] [Google Scholar]
17. O'Brien CM, Holmes B, Faucett S, Zhang LG. Three-dimensional printing of nanomaterial scaffolds for complex tissue regeneration. Tissue Eng Part B Rev 2015;21:103–114 [PMC free article] [PubMed] [Google Scholar]
O'Brien CM, Holmes B, Faucett S, Zhang LG. Three-dimensional printing of nanomaterial scaffolds for complex tissue regeneration. Tissue Eng Part B Rev 2015;21:103–114 [PMC free article] [PubMed] [Google Scholar]
18. Fedorovich NE, Schuurman W, Wijnberg HM, et al.. Biofabrication of osteochondral tissue equivalents by printing topologically defined, cell-laden hydrogel scaffolds. Tissue Eng Part C Methods 2011;18:33–44 [PMC free article] [PubMed] [Google Scholar]
19. Butscher A, Bohner M, Hofmann S, et al.. Structural and material approaches to bone tissue engineering in powder-based three-dimensional printing. Acta Biomater 2011;7:907–920 [PubMed] [Google Scholar]
20. Cima MJ, Haggerty JS, Sachs EM, Williams PA, inventors; Massachusetts Institute of Technology, Cambridge, Massachusetts, assignee. Three-dimensional printing techniques. Patent US5204055 A. 1993 [Google Scholar]
21. Butscher A, Bohner M, Roth C, et al.. Printability of calcium phosphate powders for three-dimensional printing of tissue engineering scaffolds. Acta Biomater 2012;8:373–385 [PubMed] [Google Scholar]
Acta Biomater 2012;8:373–385 [PubMed] [Google Scholar]
22. Seitz H, Deisinger U, Leukers B, et al.. Different calcium phosphate granules for 3-D printing of bone tissue engineering scaffolds. Adv Eng Mater 2009;11:B41–B46 [Google Scholar]
23. Castilho M, Moseke C, Ewald A, et al.. Direct 3D powder printing of biphasic calcium phosphate scaffolds for substitution of complex bone defects. Biofabrication 2014;6:015006. [PubMed] [Google Scholar]
24. Becker ST, Bolte H, Krapf O, et al.. Endocultivation: 3D printed customized porous scaffolds for heterotopic bone induction. Oral Oncol 2009;45:e181–e188 [PubMed] [Google Scholar]
25. Warnke PH, Seitz H, Warnke F, et al.. Ceramic scaffolds produced by computer-assisted 3D printing and sintering: characterization and biocompatibility investigations. J Biomed Mater Res Part B Appl Biomater 2010;93:212–217 [PubMed] [Google Scholar]
26. Vorndran E, Klarner M, Klammert U, et al.. 3D powder printing of β-tricalcium phosphate ceramics using different strategies. Adv Eng Mater 2008;10:B67–B71 [Google Scholar]
Adv Eng Mater 2008;10:B67–B71 [Google Scholar]
27. Butscher A, Bohner M, Doebelin N, et al.. Moisture based three-dimensional printing of calcium phosphate structures for scaffold engineering. Acta Biomater 2013;9:5369–5378 [PubMed] [Google Scholar]
28. Khalyfa A, Vogt S, Weisser J, et al.. Development of a new calcium phosphate powder-binder system for the 3D printing of patient specific implants. J Mater Sci Mater Med 2007;18:909–916 [PubMed] [Google Scholar]
29. Al-Munajjed AA, Plunkett NA, Gleeson JP, et al.. Development of a biomimetic collagen-hydroxyapatite scaffold for bone tissue engineering using a SBF immersion technique. J Biomed Mater Res Part B Appl Biomater 2009;90:584–591 [PubMed] [Google Scholar]
30. Dutta Roy T, Simon JL, Ricci JL, et al.. Performance of hydroxyapatite bone repair scaffolds created via three-dimensional fabrication techniques. J Biomed Mater Res Part A 2003;67A:1228–1237 [PubMed] [Google Scholar]
31. Tarafder S, Balla VK, Davies NM, et al. . Microwave-sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering. J Tissue Eng Regen Med 2013;7:631–641 [PMC free article] [PubMed] [Google Scholar]
. Microwave-sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering. J Tissue Eng Regen Med 2013;7:631–641 [PMC free article] [PubMed] [Google Scholar]
32. Suwanprateeb J, Sanngam R, Suvannapruk W, Panyathanmaporn T. Mechanical and in vitro performance of apatite–wollastonite glass ceramic reinforced hydroxyapatite composite fabricated by 3D-printing. J Mater Sci Mater Med 2009;20:1281–1289 [PubMed] [Google Scholar]
33. Gbureck U, Hölzel T, Klammert U, et al.. Resorbable dicalcium phosphate bone substitutes prepared by 3D powder printing. Adv Funct Mater 2007;17:3940–3945 [Google Scholar]
34. Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert Rev Med Devices 2006;3:49–57 [PubMed] [Google Scholar]
35. Klammert U, Gbureck U, Vorndran E, et al.. 3D powder printed calcium phosphate implants for reconstruction of cranial and maxillofacial defects. J Craniomaxillofac Surg 2010;38:565–570 [PubMed] [Google Scholar]
36.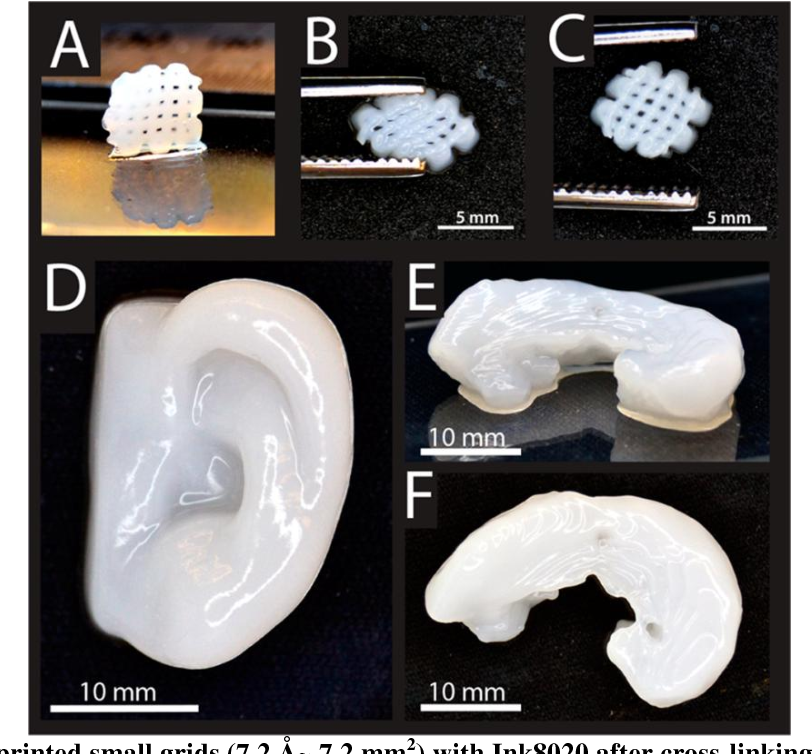 Tada H, Hatoko M, Tanaka A, et al.. Preshaped hydroxyapatite tricalcium-phosphate implant using three-dimensional computed tomography in the reconstruction of bone deformities of craniomaxillofacial region. J Craniofac Surg 2002;13:287–292 [PubMed] [Google Scholar]
Tada H, Hatoko M, Tanaka A, et al.. Preshaped hydroxyapatite tricalcium-phosphate implant using three-dimensional computed tomography in the reconstruction of bone deformities of craniomaxillofacial region. J Craniofac Surg 2002;13:287–292 [PubMed] [Google Scholar]
37. Temple JP, Hutton DL, Hung BP, et al.. Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J Biomed Mater Res Part A 2014;102:4317–4325 [PubMed] [Google Scholar]
38. Grayson WL, Frohlich M, Yeager K, et al.. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci USA 2010;107:3299–3304 [PMC free article] [PubMed] [Google Scholar]
39. Becker ST, Douglas T, Acil Y, et al.. Biocompatibility of individually designed scaffolds with human periosteum for use in tissue engineering. J Mater Sci Mater Med 2010;21:1255–1262 [PubMed] [Google Scholar]
40. Inzana JA, Olvera D, Fuller SM, et al.. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014;35:4026–4034 [PMC free article] [PubMed] [Google Scholar]
Biomaterials 2014;35:4026–4034 [PMC free article] [PubMed] [Google Scholar]
41. Detsch R, Schaefer S, Deisinger U, et al.. In vitro: osteoclastic activity studies on surfaces of 3D printed calcium phosphate scaffolds. J Biomater Appl 2011;26:359–380 [PubMed] [Google Scholar]
42. Konopnicki S, Sharaf B, Resnick C, et al.. Tissue-Engineered Bone With 3-Dimensionally Printed β-Tricalcium Phosphate and Polycaprolactone Scaffolds and Early Implantation: An In Vivo Pilot Study in a Porcine Mandible Model. J Oral Maxillofac Surg 2015;73:1016.e1–1016.e11 [PubMed] [Google Scholar]
43. Suwanprateeb J, Sanngam R, Suwanpreuk W. Fabrication of bioactive hydroxyapatite/bis-GMA based composite via three dimensional printing. J Mater Sci Mater Med 2008;19:2637–2645 [PubMed] [Google Scholar]
44. Lim JY, Loiselle AE, Lee JS, et al.. Optimizing the osteogenic potential of adult stem cells for skeletal regeneration. J Orthopaed Res 2011;29:1627–1633 [PMC free article] [PubMed] [Google Scholar]
45. Liu X, Lim JY, Donahue HJ, et al.. Influence of substratum surface chemistry/energy and topography on the human fetal osteoblastic cell line hFOB 1.19: phenotypic and genotypic responses observed in vitro. Biomaterials 2007;28:4535–4550 [PMC free article] [PubMed] [Google Scholar]
Liu X, Lim JY, Donahue HJ, et al.. Influence of substratum surface chemistry/energy and topography on the human fetal osteoblastic cell line hFOB 1.19: phenotypic and genotypic responses observed in vitro. Biomaterials 2007;28:4535–4550 [PMC free article] [PubMed] [Google Scholar]
46. Loiselle AE, Wei L, Faryad M, et al.. Specific biomimetic hydroxyapatite nanotopographies enhance osteoblastic differentiation and bone graft osteointegration. Tissue Eng Part A 2013;19:1704–1712 [PMC free article] [PubMed] [Google Scholar]
47. Antolino NE, et al.. Lost mold rapid infiltration forming of mesoscale ceramics: part 1, fabrication. J Am Ceramic Soc 2009;92.s1:S63–S69 [PMC free article] [PubMed] [Google Scholar]
48. Antolino N. Lost mold-rapid infiltration forming: strength control in mesoscale 3Y-TZP ceramics. Dissertation. The Pennsylvania State University, University Park, PA, 2010 [Google Scholar]
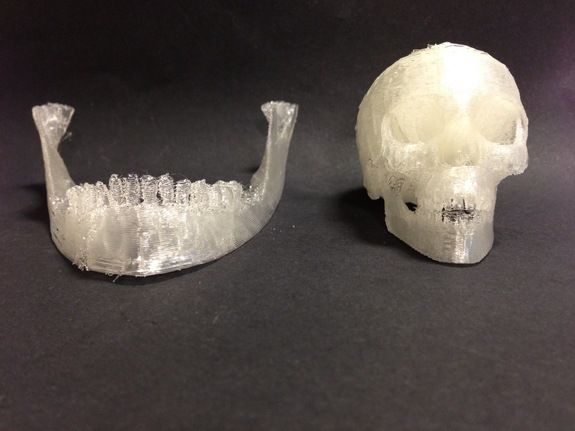
3D printed implants made from a mixture of bone powder and plastic could be ideal for bone replacement
Many people require surgery to replace the bones of the skull and face - usually a fragment of the patient's own legs is used during this procedure. However, the results often leave much to be desired, not to mention possible complications. Fortunately, there is a lot of research being done on alternative methods of making bones that are ideal for a particular site. We are talking about 3D printing of partially plastic structures on which living cells can be placed. However, the difficulty is to stimulate the cells to form bone tissue - for this, scientists from Johns Hopkins University used bone powder.
However, the results often leave much to be desired, not to mention possible complications. Fortunately, there is a lot of research being done on alternative methods of making bones that are ideal for a particular site. We are talking about 3D printing of partially plastic structures on which living cells can be placed. However, the difficulty is to stimulate the cells to form bone tissue - for this, scientists from Johns Hopkins University used bone powder.
Scientists have been working on 3D printing plastic implants – in this case, polycaprolactone, a biodegradable material with a low melting point (80-100 degrees). This plastic provides the necessary strength, but is not able to stimulate cell growth - so the researchers used it in combination with powdered bone material obtained from cows. It is bone powder that contains structural proteins that allow stem cells to form bone tissue. In addition, according to scientists, the powder makes the plastic rougher, which helps to fix cells and enhance growth factors.
To evaluate the potential of the composite material, the scientists tested various combinations of bone powder and polycaprolactone. The material with the maximum bone content, 85%, was not suitable for 3D printing at all - the structures simply fell apart. As for the other combinations that were printed, they were evaluated in terms of their ability to stimulate cell growth. For this, stem cells extracted from the patient's adipose tissue during liposuction were used in the construction.
After three weeks, the scientists evaluated the results - the greatest progress towards transformation into bone tissue was shown by an implant with a bone powder content of 70%. Samples with a bone content of 30% also showed good results in all tests, although not as impressive - so the researchers decided to conduct an experiment in real conditions.
During the experiment, scientists transplanted 3D-printed bone implants into the skulls of mice, in which holes were made that were unable to heal on their own. After some time, the mice underwent computed tomography, which showed that the material with a content of 30% and 70% bone powder led to much faster bone development compared to pure polycaprolactone implants.
After some time, the mice underwent computed tomography, which showed that the material with a content of 30% and 70% bone powder led to much faster bone development compared to pure polycaprolactone implants.
Experiments with various combinations are still ongoing - scientists intend to determine their advantages and disadvantages, as well as to conduct tests with human bones and other improvements that, in particular, can stimulate the formation of new blood vessels.
Tags: 3D printing, implant, Johns Hopkins University, polycaprolactone, 3D printer
Biomaterial for 3D printing of temporary human bones created Photo: Adam E. Jakus
Attempts to create materials for 3D printing of temporary human bones (osteoregenerative biomaterials) have been made repeatedly. Unfortunately, they still suffer from a number of shortcomings. Among them are the inability to quickly and accurately reproduce a new bone, high cost and limited production capabilities, and the complexity of processing during a surgical operation.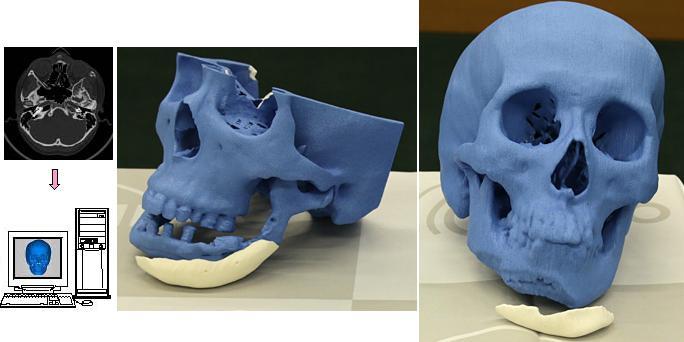
The new biomaterial is devoid of all these shortcomings. If all tests are successful, then in a few years people will be able to get strong, superelastic and cheap artificial bones that will biodegrade in the body in a few years (they are gradually replaced by natural bone tissue). The most interesting thing is that the material extrusion process at room temperature, theoretically, allows printing bones even on home printers.
As the scientists write, the new material HB (from hyperelastic “bone”) consists of 90% by weight of hydroxyapatite and 10% of polycaprolactone.
Hydroxylapatite Ca 10 (PO 4 ) 6 (OH) 2 is the main mineral constituent of natural human bones. In most bones, it is about 50% of the total mass, and in tooth enamel - 96%. In medicine, a synthetic analog has long been used in traumatology, orthopedics, and bone surgery as a filler that replaces parts of a lost bone. In dentistry, it is also used in toothpastes as a remineralizing element that strengthens tooth enamel.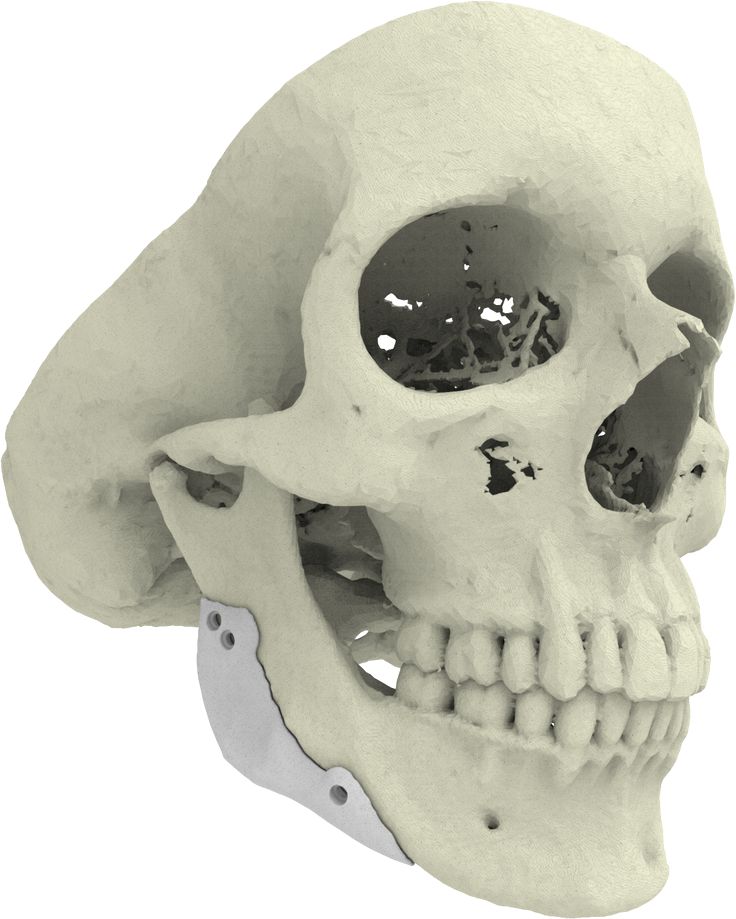
But polycaprolactone (PCL) is not found in biological materials. It is a biodegradable polyester, which is used in industry for the production of polyurethanes. It is made into biodegradable bags. In medicine, PCL is also used as a suture material and as a self-absorbable long-acting thermal implant (filler), which has the ability to stimulate the growth of fibrous tissue and replenish volume due to its own components. Many tablets are produced in PCL capsules, they are absorbed in the body. In addition, PCL is used in mass 3D printing as a material for prototyping. By its properties, viscous PCL is similar to a natural resin like gutta-percha.
Experiments have shown that artificial bones of this composition can be quickly printed at room temperature at a speed of up to 275 cm 3 /hour by extrusion, that is, by forcing a viscous melt of material or a thick paste through a forming hole. A special solvent is used to create a viscous mixture that is loaded into the printer.
Printing an implant with conventional extrusion at room temperature is a big advantage because other bone implants are created at high temperature using lasers, explains Ramille Shah, lead author and research team leader at Northwestern University ( USA). During the experiments, the researchers themselves used a commercially available 3D-Bioplotter System printer manufactured by EnvisionTec. This device can be purchased for between $250,000 and $300,000.
Of course, in the household, such a printer would be too expensive. But every hospital or surgical center can afford it. In principle, even private individuals can buy such printers and print new bones for themselves or pets if necessary (of course, it is advisable to turn to a professional surgeon for the operation).
EnvisionTEC 3D-Bioplotter
Artificial bones demonstrate good mechanical properties: strain to failure from 32% to 67%, elastic modulus from 4 to 11 MPa. Thus, it is an elastic and durable material. It is also characterized by high absorbency (porosity 50%), supports the viability and spread of living cells. Tests have shown that the material does not interfere with the formation of bone marrow cells from mesenchymal stem cells.
It is also characterized by high absorbency (porosity 50%), supports the viability and spread of living cells. Tests have shown that the material does not interfere with the formation of bone marrow cells from mesenchymal stem cells.
So far, the biocompatibility of HB has only been tested in laboratory animals, but these experiments have shown great promise. Subcutaneous implants in mice did not cause rejection within 7-35 days. In rats, a bone graft was placed in the posterolateral part of the spine (posterior lateral fusion) for 8 weeks, and new bone formation was recorded. We also conducted an experiment on a primate with skull damage (4 weeks).
During all experiments, HB did not cause a negative reaction of the immune system. At the same time, normal vascularization (formation of blood vessels) was noted, the artificial bone quickly integrated into the surrounding tissues, quickly ossified and supported the growth of new bone tissue without additional intervention.