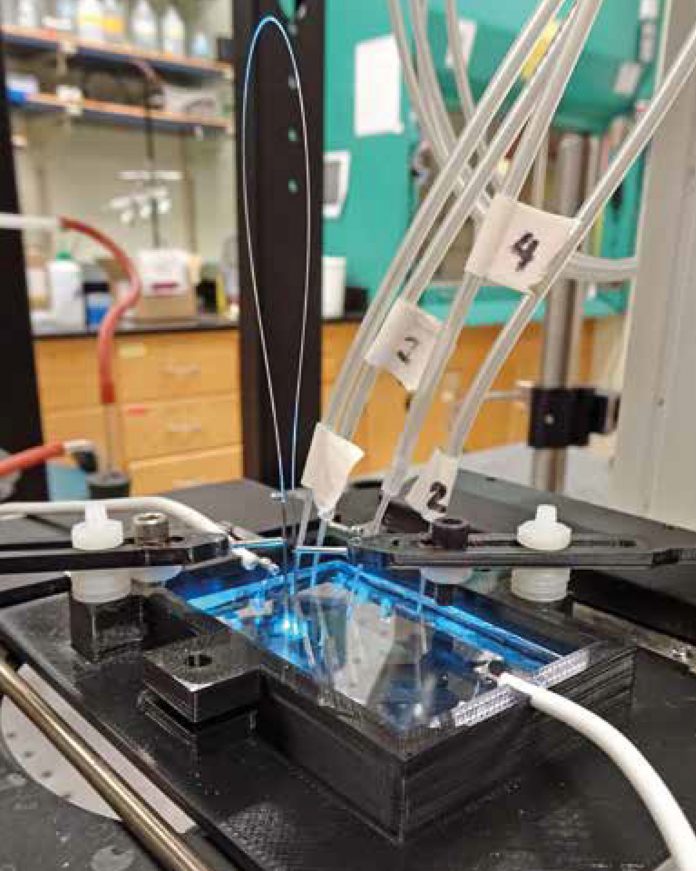
3D printed microfluidic automation
Folch Lab Microtechnology Projects
Microtechnology Projects
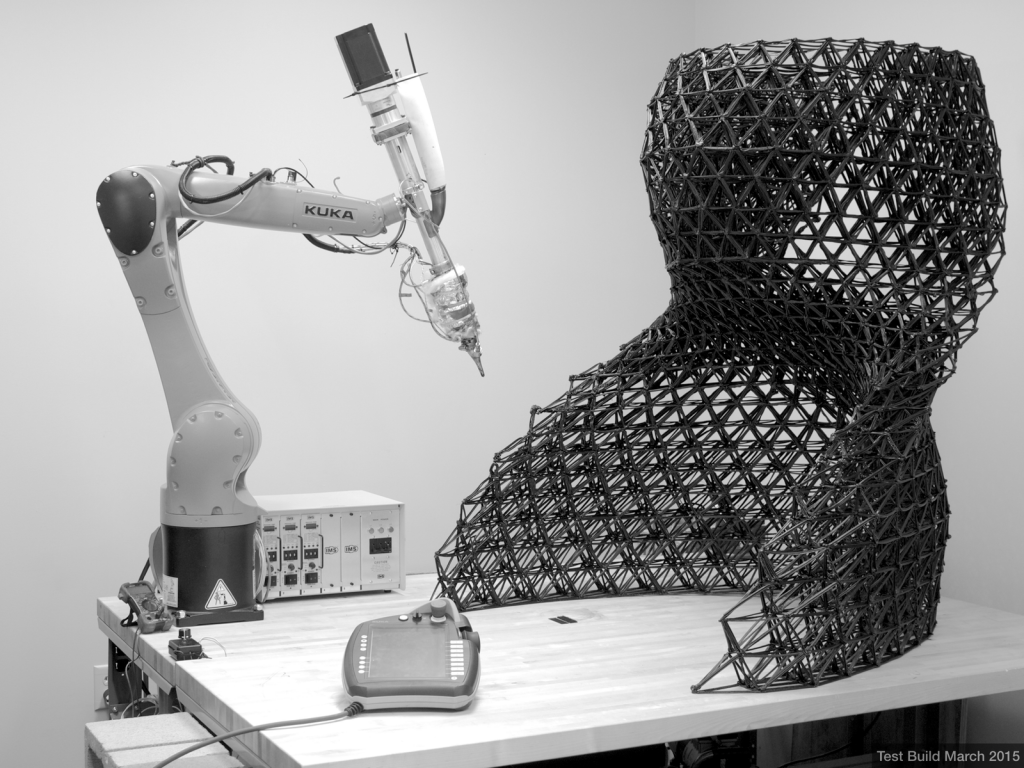
3D-Printing
The “3D-printing revolution” is now embracing the range of feature sizes and materials that are starting to appeal to microfluidic engineers and users. There are multiple features of 3D-printing technology that make it very attractive when compared to PDMS or plastic molding. Using a simple desktop computer and prior to fabrication, the design is digitally built in all 3D detail as a 3D CAD file, can be assembled in modules by remotely collaborating teams, digitally inspected in 3D, and the mechanical and fluidic behavior of the device can be inexpensively simulated using finite-element modeling. Structures are created by adding materials, without requiring any etching or dissolution, so processing is environmentally and economically efficient. By contrast, PDMS and plastic molding are limited to layered designs, where each layer must be processed separately and bonded; knowledge of the final 3D structures prior to fabrication is not realistic and collaborative design between different teams is impractical. Interestingly, some 3D-printing approaches are now becoming more affordable than PDMS molding due to the availability of low-cost desktop printers and supplies. Last but not least, the inherently low throughput of PDMS molding and the high-cost of plastic molding have made it difficult to launch microfluidic device companies; 3D printing technologies offer a natural path from prototype to commercialization that should engage many ongoing translational efforts in microfluidics. In biomedicine, shortening the time from prototype to product enables personalized devices, accelerates R&D, and helps reduce the cost of access to healthcare. Thus, we predict that in the next few years most PDMS and plastic molding activities in academia will have been replaced by 3D-printing.
References:
A Naderi, N Bhattacharjee, A Folch, "Digital Manufacturing for Microfluidics", Annual review of biomedical engineering 21: 325-36 (2019).
AP Kuo, N Bhattacharjee, YS Lee, K Castro, YT Kim, A Folch, "High‐Precision Stereolithography of Biomicrofluidic Devices", Advanced Materials Technologies 4: 1800395 (2019).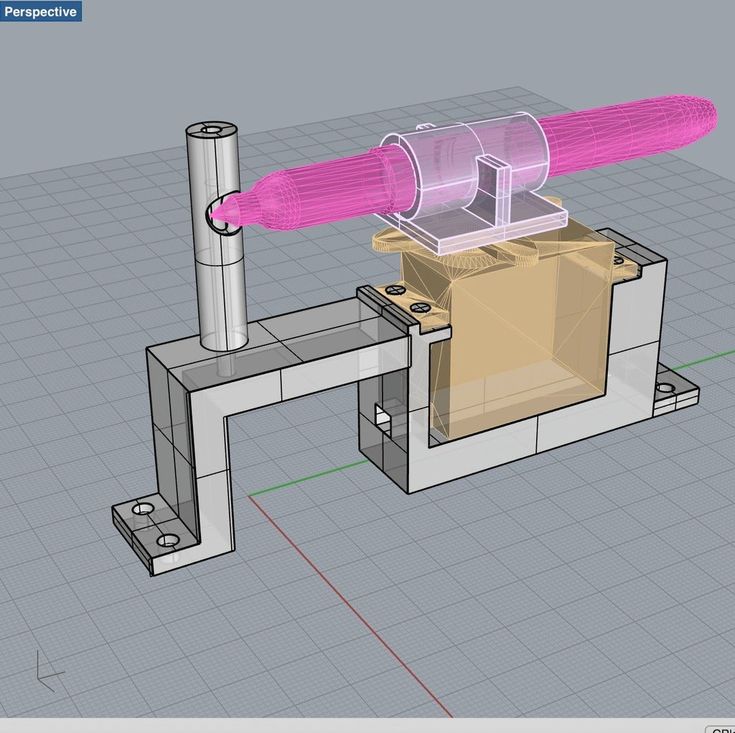
YT Kim, S Bohjanen, N Bhattacharjee, A Folch, "Partitioning of hydrogels in 3d-printed microchannels", Lab on a Chip 19: 3086-3093 (2019).
YS Lee, N Bhattacharjee, A Folch, "3D-printed Quake-style microvalves and micropumps", Lab on a Chip 18: 1207-1214 (2018).
Y.T. Kim, K. Castro, and A. Folch, “Digital Manufacturing of Selective Porous Barriers in Microchannels Using Multi-Material Stereolithography”, Micromachines 9: 125 (2018).
N. Bhattacharjee, C. Parra-Cabrera, Y.T. Kim, A.P. Kuo, and A. Folch, “Desktop-Stereolithography 3D-Printing of a Poly(dimethylsiloxane)-based Material with Sylgard-184 Properties, Advanced Materials, 1800001 (2018).
AK Au, W Huynh, LF Horowitz, A Folch, "3D‐printed microfluidics", Angewandte Chemie International Edition 55: 3862-3881 (2016).
A. Urrios, C. Parra-Cabrera, A. M. Gonzalez-Suarez, N. Bhattacharjee, L. G. Rigat-Brugarolas, U. Nallapatti, J. Samitier, C. A. DeForest, F.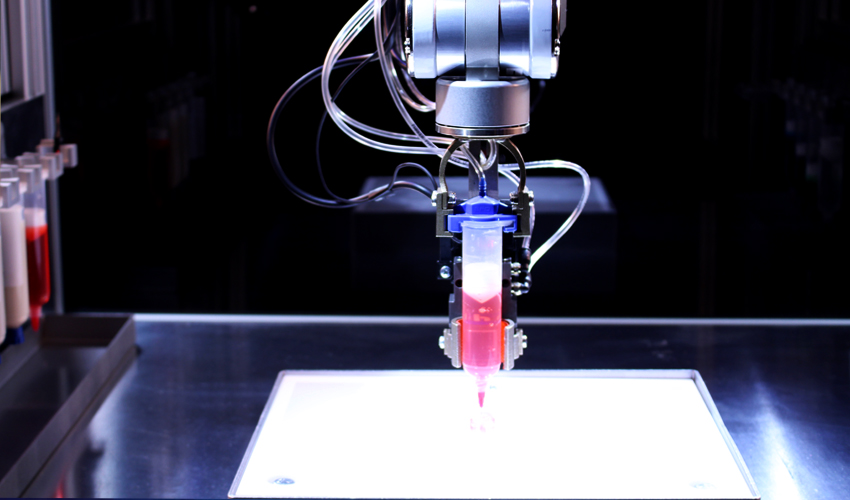 Posas, J. L. Garcia-Cordero, and A. Folch, “3D-Printing of transparent bio-microfluidic devices in PEG-DA”, Lab on a Chip 16: 2287 (2016).
Posas, J. L. Garcia-Cordero, and A. Folch, “3D-Printing of transparent bio-microfluidic devices in PEG-DA”, Lab on a Chip 16: 2287 (2016).
N Bhattacharjee, A Urrios, S Kang, A Folch, "The upcoming 3D-printing revolution in microfluidics", Lab on a Chip 16: 1720-1742 (2016). [COVER]
A.K. Au, N. Bhattacharjee, L. Horowitz, T. Chang, and A. Folch, “3D-printed microfluidic automation”, Lab on a Chip 15: 1934 (2015).
W. Lee, D. Kwon, B. Chung, G.Y. Jung, A. Au, A. Folch, and S. Jeon, "Ultrarapid detection of pathogenic bacteria using a 3D immunomagnetic flow assay", Anal. Chem. 86: 6683 (2014).
A.K. Au, W. Lee, and A. Folch, "Mail-order microfluidics: evaluation of stereolithography for the production of microfluidic devices", Lab on a Chip 14: 1294 (2014).
Cellular Micropatterns
Tissue function is modulated by the spatial organization of cells on a sub-millimeter scale. For this reason, artificial replication of cellular microstructures is important in understanding, measuring, and simulating their in vivo functions in the laboratory.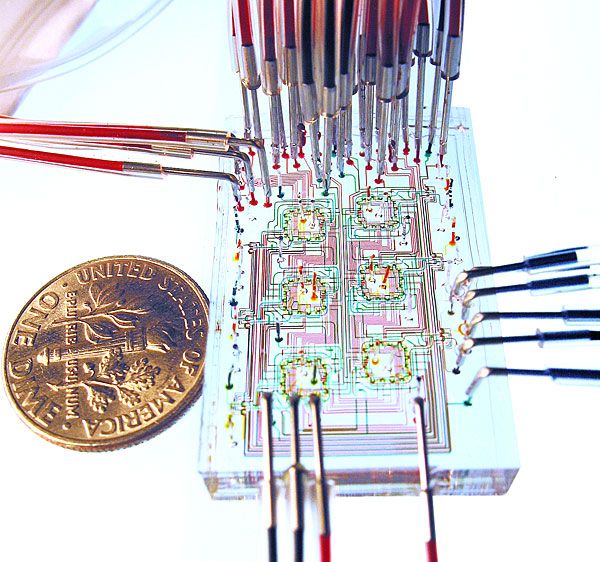 We are continuously developing techniques that allow us to selectively attach cells on a variety of substrates, including biocompatible polymers, hydrogels, and porous substrates.
We are continuously developing techniques that allow us to selectively attach cells on a variety of substrates, including biocompatible polymers, hydrogels, and porous substrates.
References:
J.W. Cheng, C.G. Sip, P.R. Lindstedt, R. Boitano, B.M. Bluestein, L.J. Gamble, and A. Folch, “'Chip-on-a-Transwell' Devices for User-Friendly Control of the Microenvironment of Cultured Cells”, ACS Applied Bio Materials 2: 4998 (2019).
Ellen Tenstad, Anna Tourovskaia, A. Folch, Ola Myklebost, and Edith Rian, “Extensive adipogenic and osteogenic differentiation of patterned human mesenchymal stem cells in a microfluidic device”, Lab Chip 10: 1401 (2010).
Rettig, J. R. and Folch, A. “Large-Scale Single-Cell Trapping and Imaging Using Microwell Arrays”, Analytical Chemistry 77: 5628-5634 (2005).
Tourovskaia, A., Figueroa-Masot, X. and Folch, A., “Differentiation-on-a-chip: A Microfluidic Platform for Long-Term Cell Culture Studies”, Lab on a Chip 5:14 (2005).
Li, N., Tourovskaia, A., and Folch, A. “Biology on a Chip: Microfabrication in Cell Culture Studies”, Critical Reviews in Biomedical Engineering 31:68 (2003).
Tourovskaia, A., Barber, T., Wickes, B., Hirdes, D., Grin, B., Castner, D. G., Healy, K. E., and Folch, A. “Micropatterns of Chemisorbed Cell Adhesion-Repellent Films Using Oxygen Plasma Etching and Elastomeric Masks”, Langmuir 19:4754 (2002).
Folch, A. and Toner, M. “Microengineering of Cellular Interactions”, Annual Rev. of Biomedical Engineering 2: 227 (2000).
Microfluidic automation
With a few exceptions, microfluidic devices are notoriously difficult to use. At best, they are based on non-intuitive user interfaces and complex tubing setups that are prone to bubble nucleation and human error. We believe that, in order to become a standard technology in clinical devices, microfluidic automation should draw some inspiration from car automation standards, whereby the user operates an intuitive user interface that commands a computer (hidden from the user), which in turns drives a car -- the user no longer drives the car.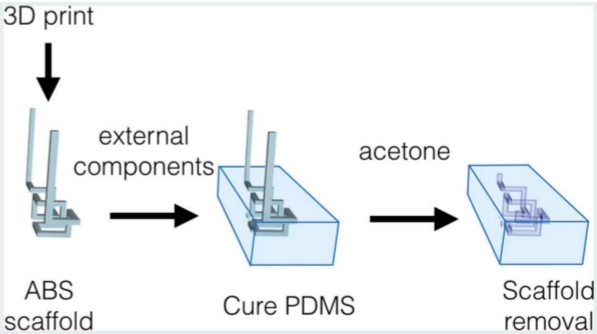 We are developing microfluidic systems where all the layers of automation (microvalves and micropumps) are hidden from the user, they can be programmed via a battery-operated mini-processor, and the user can introduce all the fluids into the device via intuitive interfaces such as a multi-well plate and standardized connectors.
We are developing microfluidic systems where all the layers of automation (microvalves and micropumps) are hidden from the user, they can be programmed via a battery-operated mini-processor, and the user can introduce all the fluids into the device via intuitive interfaces such as a multi-well plate and standardized connectors.
References:
Adina Scott, Anthony K. Au, Elise Vinckenbosch, and Albert Folch, “A microfluidic D-subminiature connector”, accepted to Lab on a Chip (2013).
A. K. Au, H. Lai, B. R. Utela, and A. Folch, “Microvalves and Micropumps for BioMEMS”, Micromachines 2, 179 (2011).
H. Lai and A. Folch, "Design and characterization of "single-stroke" peristaltic PDMS micropumps", Lab Chip 11, 336 (2011).
J. M. Hoffman, M. Ebara, J. J. Lai, A. S. Hoffman, A. Folch, and P. Stayton, "A helical flow, circular microreactor for separating and enriching smart polymer-antibody capture reagents", Lab Chip 10, 3130 (2010).
Lam, E.W., Cooksey, G.A., Finlayson, B.A., and Folch, A. “Microfluidic Circuits with Tunable Flow Resistances”, Appl. Phys. Lett. 89: 164105 (2006).
Hsu, C.-H. and Folch, A. “Spatiotemporally-Complex Concentration Profiles Using a Tunable Chaotic Micromixer”, Appl. Phys. Lett. 89: 144102 (2006).
Frevert, C.W., Boggy, G., Keenan, T.M., and Folch, A. “Measurement of cell migration in response to an evolving radial chemokine gradient triggered by a microvalve”, Lab on a Chip 6: 849 (2006).
Li, N., Hsu, C.-H., and Folch, A. “Parallel mixing of Photolithographically-Defined Nanoliter Volumes Using Elastomeric Microvalve Arrays”, Electrophoresis 26: 3758-3764 (2005).
Hsu, C.-H. and Folch, A. “Microfluidic Devices with Tunable Microtopographies”, Applied Physics Letters 86, 023508 (2005).
Hoffman, J., Shao, J., Hsu, C.-H., and Folch, A. “Elastomeric Molds with Tunable Microtopographies”, Advanced Materials 16:2201 (2004).
Gradient Generators
Biomolecule gradients have been shown to play roles in a wide range of biological processes including development, inflammation, wound healing, and cancer metastasis. Elucidation of these phenomena requires the ability to expose cells to biomolecule gradients that are quantifiable, controllable, and mimic those that are present in vivo. Microfluidic gradient generators offer greater levels of precision, quantitation, and spatiotemporal gradient control than traditional methods, and may greatly enhance our understanding of many biological phenomena.
References:
C. G. Sip, N. Bhattacharjee, and A. Folch, “A Modular Cell Culture Device for Generating Arrays of Gradients Using Stacked Microfluidic Flows”, Biomicrofluidics 5, 022210 (2011).
D. M. Cate, C. G. Sip, and A. Folch, "A microfluidic platform for generation of sharp gradients in open-access culture", Biomicrofluidics 4, 044105 (2010).
Keenan, T. M., Frevert, C.W., Wu, A., Wong, V., and Folch, A. “A New Method for Studying Gradient-Induced Neutrophil Desensitization Based on an Open Microfluidic Chamber”, Lab Chip 10: 116 (2010).
M., Frevert, C.W., Wu, A., Wong, V., and Folch, A. “A New Method for Studying Gradient-Induced Neutrophil Desensitization Based on an Open Microfluidic Chamber”, Lab Chip 10: 116 (2010).
Bhattacharjee, N., Li, N., Keenan, T.M., and Folch, A. “A Neuron-Benign Microfluidic Gradient Generator for Studying the Growth of Mammalian Neurons towards Axon Guidance Factors”, Integrative Biology 2, 669 (2010).
Keenan, T.M. and Folch, A. “Biomolecular gradients in cell culture systems”, Lab Chip 8, 34 (2008).
Hsu, C.-H. and Folch, A. “Spatiotemporally-Complex Concentration Profiles Using a Tunable Chaotic Micromixer”, Appl. Phys. Lett. 89: 144102 (2006).
Neils, C. M., Tyree, Z., Finlayson, B., and Folch, A. “Combinatorial Mixing of Microfluidic Streams”, Lab on a Chip 4:342 (2004).
Chen, C., Hirdes, D., and Folch, A. “Gray-Scale Photolithography Using Microfluidic Photomasks”, Proceedings of National Academy of Sciences 100:1499 (2003).
3D Printing and Microfluidics - 3DHeals
“Microfluidics refers to the behavior, precise control, and manipulation of fluids that are geometrically constrained to a small scale (typically sub-millimeter) at which surface forces dominate volumetric forces…It has practical applications in the design of systems that process low volumes of fluids to achieve multiplexing, automation, and high-throughput screening.” (Wikipedia)
However, most of you are already very familiar with the concept of microfluidics, often appearing as a transparent plastic chip with strategically designed channels and controlled fluid dynamics to manipulate cells or event organoids. The concept of “organ-on-a-chip” is the more popular application in the media. The infamous Theranos touted it as its central technology, which is still considered a myth by experts (we will see). However, many biotechnology companies are now employing microfluidics at scale, and its ecosystem is maturing rapidly. Lately, 3D printing has been both a manufacturing method and an application for microfluidics. Better microfabrication techniques, new biomaterials, and expanded design capabilities add acceleration to its latest growth.
Lately, 3D printing has been both a manufacturing method and an application for microfluidics. Better microfabrication techniques, new biomaterials, and expanded design capabilities add acceleration to its latest growth.
In this upcoming webinar, we are fortunate to have diverse academic and industrial experts who will share with us their latest research, industry insights, trends, and entrepreneurial activities.
Subscribe here to receive event emails.
Speakers:
Jan Pal-Goetzen is the Founder and CEO of 3D printed microTEC ,Bethesda Maryland. The company takes 3D printing beyond rapid prototyping. While pushing the resolution limits down to 1μm lateral, the unique batch approach allows mass volume production on a single 3D-printer. The solutions are always innovative and often groundbreaking in the field. Jan has been in the field of 3D printed microfluidics and packaging of microelectronics for over 10 years. He holds a PhD in Physics from Marburg University Germany.
An experienced Chartered Mechanical Engineer, Paul is an engineering consultant specialising in Life Sciences, and co-founder and CEO of Rapid Fluidics. His work focuses on the design and rapid-prototyping of microfluidic systems but also covers a wide range of other projects from medical device design through to technical reviews of water, sanitation and health projects for the Bill and Melinda Gates Foundation.
Cory Lambertson has over 8 years of experience in additive manufacturing. He started his career in the Dental Laboratory Industry after college by joining his Father at Heartland Dental Laboratory in 2013. From there, Cory ventured off into corporate dentistry which is where he was first introduced to the Asiga brand of 3D Printers. Cory found a passion for the Asiga brand of 3D Printers due to the revolutionary changes that they bring to the dental industry. Today, Cory is the General Manager of Americas at Asiga, and is based out of Ann Arbor Michigan.
Gavin D. M. Jeffries is a Founder and Chief Technology Officer at Fluicell AB. Gavin holds a PhD in Chemistry from the University of Washington and is a former assistant Professor at Chalmers University of Technology. Gavin has published over 40 peer-reviewed publications with a cumulative citation count of over three thousand. As an entrepreneur/founder of two biotech and optics companies and inventor of multiple patents and technologies, Gavin has a strong background in microfluidics, single-cell analysis, and optical platform integration. Fluicell AB is a Life-Science tool company with a commercialized product portfolio of microfluidic products to investigate individual cells, primarily in the fields of drug development and regenerative medicine. Fluicell recently developed a unique high-resolution technology for bioprinting in both 2D and 3D under the name Biopixlar®. Built upon Fluicell’s patented microfluidic technology, Biopixlar can generate detailed, multi-cellular biological tissues, directly in cell culture media, without the use of a bioink.
M. Jeffries is a Founder and Chief Technology Officer at Fluicell AB. Gavin holds a PhD in Chemistry from the University of Washington and is a former assistant Professor at Chalmers University of Technology. Gavin has published over 40 peer-reviewed publications with a cumulative citation count of over three thousand. As an entrepreneur/founder of two biotech and optics companies and inventor of multiple patents and technologies, Gavin has a strong background in microfluidics, single-cell analysis, and optical platform integration. Fluicell AB is a Life-Science tool company with a commercialized product portfolio of microfluidic products to investigate individual cells, primarily in the fields of drug development and regenerative medicine. Fluicell recently developed a unique high-resolution technology for bioprinting in both 2D and 3D under the name Biopixlar®. Built upon Fluicell’s patented microfluidic technology, Biopixlar can generate detailed, multi-cellular biological tissues, directly in cell culture media, without the use of a bioink. This all-in-one discovery platform helps researchers around the globe to build biological tissues for drug development, disease understanding and regenerative medicine research.
This all-in-one discovery platform helps researchers around the globe to build biological tissues for drug development, disease understanding and regenerative medicine research.
Yang Xu is a postdoctoral researcher at the Center for Advanced Manufacturing, University of Southern California. He earned an M.S. degree in Computer Science and a Ph.D. degree from USC in 2021. His research interests are novel 3D printing processes for polymers, polymer-based composites, and related applications. His recent work, “In-situ Transfer Vat Photopolymerization for Transparent Microfluidic Device Fabrication”, solved the z-resolution challenge of 3D printing microfluidic devices.
Moderator:
Dr. Jenny Chen is trained as a neuroradiologist, founder/CEO of 3DHEALS. Her main interests include next generation education, 3D printing in the healthcare sector, automated biology, artificial intelligence. She is an angel investor who invests in Pitch4D companies.
Subscribe here to receive event emails.
Now On-Demand
Microfluidics, Technology, Commercialization
3D printing helps create record thin microfluidic channels
A team of scientists from Brigham Young University (USA) has proposed a 3D printing method to create microfluidic laboratories-on-a-chip with record-breaking thin channels. Thanks to DLP (“digital light processing”) technology and careful selection of the printing material, the authors achieved a 100% yield when creating channels with a cross section of 18 × 20 square microns - 10 times smaller than what has been possible so far. The study was published in a specialized journal on microfluidics Lab on a Chip .
Microfluidics is the field of study of the behavior of fluids in micron-thick channels. Due to their small size, microfluidic devices make it possible to carry out complex multi-stage manipulations with liquids and objects placed in them (cells, bubbles, particles, drops) using chips several millimeters in size. Hence the name of the most popular microfluidic devices - "laboratories on a chip".
Hence the name of the most popular microfluidic devices - "laboratories on a chip".
Compared to macrosystems - the usual "pipes" and relatively thick (mm) capillaries - in microfluidics, the behavior of the liquid changes somewhat. For example, viscous resistance and surface properties play an important role. A separate problem is the production of thin channels, because, as in microelectronics, each chip consists of numerous "tracks" (channels for liquids) with "crossroads", valves and sections with a special shape and relief of the walls. nine0005 Soft lithography methods are traditionally used to create microfluidic systems, but 3D printing has also gained popularity recently. However, until now, she lacked the accuracy and resolution to seriously compete with lithography. The authors of the new work created their own modification of a commercial 3D printer and selected a specific material for printing, as a result of which they were able to achieve a record resolution.
Wall materials in microfluidics are usually polymers, which are formed from a liquid solution during photopolymerization. To do this, each layer applied during printing must be irradiated with light with a certain wavelength. In this case, the illuminated sections of the layer will freeze and become walls, and the unlit sections will then be washed away, and in this place there will be a “void”, that is, the channel itself. In order to accurately illuminate only the required areas of the layer, the authors used the method
To do this, each layer applied during printing must be irradiated with light with a certain wavelength. In this case, the illuminated sections of the layer will freeze and become walls, and the unlit sections will then be washed away, and in this place there will be a “void”, that is, the channel itself. In order to accurately illuminate only the required areas of the layer, the authors used the method
DLP
, which is widely used in projectors, both home and industrial. These devices have a system of prisms and mirrors that creates the necessary sequence of pixels in each image. The light source in the work was an LED with a wavelength of 385 nanometers, which is lower than the commonly used 405 nanometers - this allowed us to consider a larger range of materials suitable for printing.
The authors paid special attention to the light-absorbing additive, which solved one of the main problems of 3D printing of microchannels. Imagine that the printer has just printed a layer that has a void (channel) in it, and the next layer should be solid (top cover, for example). When illuminating a "pixel" located above the void, the light can penetrate the previous layer, resulting in a wall instead of a void. To avoid this, a photo-absorbing additive is added, which prevents light from penetrating further than required. nine0005 The authors selected the photoabsorbent from 20 candidates, which were compared according to 6 criteria: solubility in the base material, absorption spectrum (compatibility with a light source), critical temperature, mechanical strength after polymerization of the sample, fluorescence ability (parasitic and unnecessary in this work) and the print resolution provided, i.e. the depth of light penetration. After such selection, scientists left only one substance - NPS (2-nitrophenyl phenyl sulfide).
When illuminating a "pixel" located above the void, the light can penetrate the previous layer, resulting in a wall instead of a void. To avoid this, a photo-absorbing additive is added, which prevents light from penetrating further than required. nine0005 The authors selected the photoabsorbent from 20 candidates, which were compared according to 6 criteria: solubility in the base material, absorption spectrum (compatibility with a light source), critical temperature, mechanical strength after polymerization of the sample, fluorescence ability (parasitic and unnecessary in this work) and the print resolution provided, i.e. the depth of light penetration. After such selection, scientists left only one substance - NPS (2-nitrophenyl phenyl sulfide).
In order to test the finished method, the authors printed several typical microfluidic systems: a serpentine, as well as a channel with a large vertical aspect ratio - 25 microns wide and 3 millimeters high. Scientists also estimated the width of one "pixel" in the print plane - 7. 6 microns, and the minimum achievable channel cross section was 18 × 20 square microns. The article notes that lithography methods can also achieve lower values, but they lack many of the possibilities of three-dimensional printing, for example, creating full-fledged 3D devices in which the channels do not lie in the same plane. nine0008
6 microns, and the minimum achievable channel cross section was 18 × 20 square microns. The article notes that lithography methods can also achieve lower values, but they lack many of the possibilities of three-dimensional printing, for example, creating full-fledged 3D devices in which the channels do not lie in the same plane. nine0008
The range of applications for 3D printing today remains very wide: from
microfluidics
and printing
organs
to construction
buildings
. The range of materials used is just as great: in addition to plastic, systems for printing
with metal
or
with glass
are known.
Taras Molotilin
Found a typo? Select the fragment and press Ctrl+Enter. nine0008
Automation in FDM. What prevents us from printing 24/7?
The 24/7 production of parts using FDM technology is hampered by the high labor intensity of each action taken, as well as the need for an operator to be near the printer.
If we compare the most common modern FDM 3D printers on the Russian market with ordinary office printers, then in terms of automation level they are closer to a manual printing press than to an MFP.
The FDM 3D printer will be idle if the operator has not removed an already finished part from the worktable or if he has run out of material. In the event of a technical malfunction, the operator spends more time finding the malfunction itself than fixing it. nine0008
If the production is small (up to 10 printers, up to 1000 parts per month), then these problems are not noticeable. Typically, such a pilot plant is run by a single specialist who has a thorough knowledge of the printers entrusted to him, but even an experienced print specialist cannot work 24/7 and produce a large product range with high quality if it changes frequently (for example, in 3D printing service farms).
If a company needs to increase the productivity of its 3D printing department, then this is usually followed by an increase in the staff of operators, an increase in the number of printers, an increase in technical costs (manufacturing defects, technical and technological downtime). When deadlines are pressed, there is a need to work first in two shifts, and then in three. nine0008
When deadlines are pressed, there is a need to work first in two shifts, and then in three. nine0008
Over the past three years of running serial orders at our St. Petersburg 3D printing services farm, we have identified the most costly operations in terms of productivity and labor intensity:
requires the presence of an operator.
2. Preparation of the working area, calibration - in case of operator error, it leads to marriage, time is lost for reprinting. nine0008
3. Media Inspection - When finished during the printing process, the printer is either idle or the product is rejected.
4. The printer is idle until the operator removes the product from the worktable.
5. The printer is idle while the operator is looking for a problem or waiting for a response from technical support. The problem applies to printers whose manufacturer does not provide detailed technical documentation. A large fleet of printers requires a dedicated service engineer. nine0008
A large fleet of printers requires a dedicated service engineer. nine0008
6. An increase in the number of orders for production requires the appearance of a technician to solve routine auxiliary tasks (for example, replacement and control of the availability of material), otherwise the workload on operators increases, rejects become more frequent, equipment is idle.
7. Delegation between operator shifts often results in overproduction of parts or loss of order information. To combat this, investments in accounting systems or the hiring of additional administrative staff are required. nine0008
It follows from this:
2. An increase in the number of orders is a non-linear increase in the labor intensity of production.
3. Staff expansion - growth of non-production costs.
4. Increasing the labor intensity of production - increasing the terms of product readiness.
5. Lack of automated accounting tools - increased costs for scrap and re-sorting.
6. Shift schedule - increased staff and increased costs for marriage and regrading.
7. Round-the-clock work - increase in staff, decrease in the level of responsibility for quality.
Thus, FDM 3D printing is scaling at a significant cost and is accompanied by an increase in staff, an increase in payroll costs, and a non-linear increase in production and administrative costs. Added to this are the difficulties with organizing in-line printing, the lack of analytics and statistical data on production, material consumption, and data on equipment loading. nine0008
The reason is the lack of technical and software automation of routine actions in FDM printing.
To solve the above scaling problems, in the spring of 2020, we began the development of an automated FDM 3D printing complex.
The following development goals were identified:
1. Automate the routine removal of finished products and material replacement, eliminating the need for the constant presence of the operator near the printers. nine0008
Automate the routine removal of finished products and material replacement, eliminating the need for the constant presence of the operator near the printers. nine0008
2. Have full information about the technical condition of the printer without visual control and operator presence.
3. Ensure the scalability of production without increasing non-production costs.
4. Reduce the labor intensity of production planning, control and accounting of printed products.
5. Reduce the risk of technical failures and downtime for service and recovery. nine0008
6. Ensure compatibility with the most common thermoplastics and composites and reduce downtime for changeovers, reduce downtime between operations.
7. Collect and analyze data on the printing process and equipment status, display analytics not only for production, but also for administrative staff, including material consumption and manufacturing defects.
8. Predict project deadlines as accurately as possible by using real time to print the first samples in a batch. nine0008
9. Implement a management system with two-way exchange with third-party CRM (Bitrix24) and accounting systems (1C: UNF) for end-to-end analytics of the effectiveness of investments in the additive unit.
In this article, we present a comprehensive solution to the task of automating FDM 3D printing - the Redfab additive manufacturing hardware and software system.
Redfab 3D printer solves existing problems in the following way:
1. Automatic Selective Feeding and Media Change
The system allows you to selectively feed, remove, cut and rewind media autonomously or at the operator's command. Up to 5 D300 2.25 kg spools or up to 8 D200 1 kg spools are simultaneously connected to each print zone. Main extruder and sub extruder each have 4 slots for 1. 75+/-0.15mm bar. Having loaded the coil compartment once, the engineer may not return to the issue of refilling the material for a long time, up to 8 days of continuous printing at a flow rate of ~50 cm3/hour, or 4 days at a flow rate of ~100 cm3/hour. At the end of plastic on the coil, the system automatically switches to the next one. This uses the media specified in the print job. nine0008
75+/-0.15mm bar. Having loaded the coil compartment once, the engineer may not return to the issue of refilling the material for a long time, up to 8 days of continuous printing at a flow rate of ~50 cm3/hour, or 4 days at a flow rate of ~100 cm3/hour. At the end of plastic on the coil, the system automatically switches to the next one. This uses the media specified in the print job. nine0008
2. Automatic ejection of finished products
The part is printed on a film pressed against the work table by vacuum. After printing is completed, the film is pulled through the cooling system, the part is separated and dumped into a container. After the finished batch is reset, the printer autonomously starts the next print. Since the parts are ejected from the hot table, reheating is not required.
3. Automatic table plane calibration
4-point print plane and 20-point table calibration with correction factors entered into the control system (MESH). The milled table has no internal stresses, as it undergoes a heat treatment procedure.
The milled table has no internal stresses, as it undergoes a heat treatment procedure.
The table is thermally decoupled from the power frame of the printer, due to which it does not warp when heated up to 140°C.
Calibration is carried out automatically without the participation of a specialist.
4. Automatic print queue
The user sets the printing parameters, the control system automatically forms a queue of projects and production jobs according to the user-defined parameters. The availability and availability of material, nozzle parameters, production priorities are taken into account.
5. Automatic service operations
The control system detects faults and accidents based on the readings of the sensors, and then attempts to independently maintain and restore the printing process in cases where it is possible. A well-thought-out control system prevents false failures and gives the user flexibility in making decisions. The printing process will not be stopped if a failure occurs in the auxiliary system. Thus, the complex will try to complete the current print, after notifying the user. nine0008
The printing process will not be stopped if a failure occurs in the auxiliary system. Thus, the complex will try to complete the current print, after notifying the user. nine0008
6. Easy maintenance
Modular design, quick-change extruders and easy access to all equipment components allow periodic maintenance to be carried out quickly. You can write a request to technical support directly from the control system. When requested and authorized by the user, our technical support service can remotely connect to the control system and both carry out a complete diagnosis of the system settings and track the printing process itself thanks to the built-in video camera. Since we produce most of the components ourselves, we are ready to promptly provide the customer with spare parts. nine0008
The Redfab 3D printer is equipped with the advanced control system, which allows real-time analysis of production processes, which is indispensable for assessing the current work and making decisions on the development of FDM printing. 1. Statistics, accounting and analysis carrying out maintenance with the output of reports and comparison of periods. nine0008
1. Statistics, accounting and analysis carrying out maintenance with the output of reports and comparison of periods. nine0008
2. Production time control
The system allows you to visualize the production time on the Gantt chart and calculates the order readiness for delivery based on the actual time of printing the first copy of the print job. This allows you to predict the completion time of the entire project with high accuracy.
3. Remote monitoring of the printing process
The user gets access to the video stream from the camera located in the working area. At the beginning and at the end of printing, a control picture is taken, which allows you to track the readiness of the product and the absence of defects. In the future, we plan to automate this aspect of work, using vision algorithms. nine0008
4. Access control and accounting
Authentication system, setting group rights, setting roles (trainee, technician, operator, administrator, technical support), logging user actions in the control system. This functionality allows you to ensure that the system is closed to third parties and restrict access to production and accounting data in accordance with the client's privacy policy.
This functionality allows you to ensure that the system is closed to third parties and restrict access to production and accounting data in accordance with the client's privacy policy.
| Parameter name | Value |
|---|---|
| Print area (X/Y/Z), mm | 250*430*250 |
| Number of printing areas in 1 complex, pcs. | 3 |
| Number of extruders per print area, pcs. | 2, direct, quick release |
| Maximum extrusion temperature, °С | 450 |
| Maximum air temperature in an active heat chamber with forced circulation, °С | 80 |
| Maximum heating temperature of the working table, °С | 140 |
| Parts Cooling Systems | Material cooling at nozzle exit. Cooling of the layer over the entire print area. |
| Performance at maximum practical printing speed, cm 3 / hour | up to 150 (for ABS Standard Filamentarno) |
| Supported file format | gcode, slicer of user choice |
| Data interfaces | LAN Ethernet, USB, Wi-Fi |
Our equipment is running 24/7 on our own 3D printing farm, so we can confidently give you a 2-year warranty on your Redfab 3D printer.
To calculate the profitability of purchasing our printer, we suggest using the calculator on our website www.redfab.ru.
The calculator allows you to estimate the rate of return on investment.
For large-scale productions (from 20 units of 3D printers), we recommend considering a one-time purchase of 4 units of Redfab printers at once for leasing at a special price.

Learn more