Pharmaceutical 3d printing
3D printing - current pharmaceutical applications and future directions
72
SHARES
3D printing has the potential to revolutionise the pharmaceutical manufacturing industry; however, few 3D-printed products have been approved since the first in 2015. In this article, EPR’s Hannah Balfour explores the technologies currently being evaluated for use in the 3D printing of pharmaceuticals, and the work of key market players to develop and advance their applications from research to commercial.
The healthcare needs of the population, and the therapeutics we use to treat them, are changing. Though generics are undeniably important, there is a noticeable shift towards personalisation and customisation of treatment – stimulated by the adoption and enhancement of omics technologies in healthcare. This, in turn, is affecting the way we manufacture drugs: drawing the industry away from large-scale batch manufacturing to more continuous, non-batch and/or small-scale production efforts.
One technology that could fulfil the requirement of personalised therapies is three-dimensional (3D) printing, also known as additive manufacturing, which uses a computerised model to guide the layer-by-layer construction of a 3D shape. Its potential to disrupt the pharmaceutical industry is vast, as 3D printing technologies could enable on-demand production of products with personalised dosages, drug combinations, geometries and release characteristics, not afforded by existing conventional manufacturing technologies like tableting and encapsulation.1
The first 3D-printed pharmaceutical – Spritam® (a levetiracetam tablet) – was approved by the US Food and Drug Administration (FDA) in July 2015,2 and since then articles describing the 3D printing of pharmaceuticals have increased year on year.3 Valued at $175.19 million in 2020 and anticipated to grow to $285.17 million by 2025,4 the 3D-printed pharmaceuticals market represents a significant opportunity to those able to capitalise on its benefits and overcome its challenges.
Here, I explore the technologies currently used for additive manufacturing of therapeutics, some of the challenges facing the sector and key developments that could inform the future of 3D-printed pharmaceuticals.
Technologies applied in the 3D printing of pharmaceuticals
There are currently several examples of 3D-printed healthcare products on the market, ranging from ibuprofen hydrogels and drug delivery devices for progesterone and pseudoephedrine, to polypills such as guaifenesin and the multi-active combination of nifedipine, captopril and glipizide.5
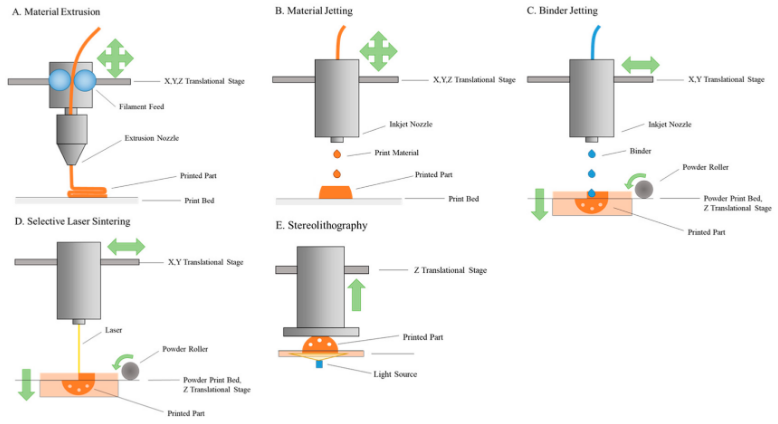
To create these products, five core techniques are currently applied: extrusion moulding printing (EMP), drop on powder (DOP) printing, selective laser sintering (SLS), stereolithography (SLA) and electrohydrodynamic 3D printing (EHD).6
Machine performing selective laser sintering, a type of 3D printing.
EMP is one of the most frequently used – sub-divided into fused deposition modelling (FDM) and semisolid extrusion moulding technology (SSE). In FDM, drug-loaded polymers are heated to a semifluid state, extruded from a printing nozzle and allowed to solidify on the printing platform, creating the desired product. Despite being a relatively cheap and operationally simple process, it is limited by the potential for active pharmaceutical ingredients (APIs) to thermally degrade during the heating process and exacerbated by low drug loading.6 SSE, conversely, uses pressure to extrude a paste through a syringe-based print head to deposit material on the printing platform. This is the method used to produce guaifenesin, a bi-layered polypill with controlled release. While SSE avoids the thermal degradation problem, its limitations include the necessity for organic solvents and complex requirements for preparing the paste, as well as the need for heavy machinery.6
In FDM, drug-loaded polymers are heated to a semifluid state, extruded from a printing nozzle and allowed to solidify on the printing platform, creating the desired product. Despite being a relatively cheap and operationally simple process, it is limited by the potential for active pharmaceutical ingredients (APIs) to thermally degrade during the heating process and exacerbated by low drug loading.6 SSE, conversely, uses pressure to extrude a paste through a syringe-based print head to deposit material on the printing platform. This is the method used to produce guaifenesin, a bi-layered polypill with controlled release. While SSE avoids the thermal degradation problem, its limitations include the necessity for organic solvents and complex requirements for preparing the paste, as well as the need for heavy machinery.6
DOP printing, or binder jetting,1 uses droplets of a binding agent from a print head to bind powder deposited on the build platform in layers into the desired product. It is relatively low cost, easy to scale up and creates tablets with high porosity, but is limited by the need for post-processing – wherein residual solvent must be eliminated and unprocessed powder recovered – low resolution and high fragility.6
It is relatively low cost, easy to scale up and creates tablets with high porosity, but is limited by the need for post-processing – wherein residual solvent must be eliminated and unprocessed powder recovered – low resolution and high fragility.6
A type of powder bed fusion called SLS is another powder-based processing 3D printing technique; however, it uses a CO2 laser to selectively sinter (heat to create solid material) selected regions of layers of powders. While its precision enables manufacturers to greatly control the microstructures of the drug products produced, SLS is low speed and has the potential to degrade drug products through the heat produced by the laser.6
3D printing has the potential to revolutionise the production of pharmaceutical products, allowing for decentralised and customised manufacturing of therapeutics”
SLA, a type of vat photopolymerisation,1 uses ultraviolet lasers to polymerise photosensitive resins in layers, repeating until the desired dosage form is created. It has the best resolution of the technologies, facilitating precise structures, and is typically used to produce oral solid dosages, hydrogels and microneedle patches for drug delivery. While it has low requirements for which chemical structures or drug/excipient properties can be used, it requires post-processing to eliminate resin toxicity, the equipment is costly, there are few approved resins for the pharmaceutical field, and efficiency is low.6
It has the best resolution of the technologies, facilitating precise structures, and is typically used to produce oral solid dosages, hydrogels and microneedle patches for drug delivery. While it has low requirements for which chemical structures or drug/excipient properties can be used, it requires post-processing to eliminate resin toxicity, the equipment is costly, there are few approved resins for the pharmaceutical field, and efficiency is low.6
EHD is an emerging technology which utilises digitally controlled deposition of materials to pattern fibrous materials, creating drug products. It enables fibre engineering on the micro-to-nano scale, allowing customised geometries and well-ordered complex structures to be formed. Researchers believe it could one day engender the small-scale manufacturing of medicines tailored to individual patient needs, such as personalised dosage form requirements or API release.6 EHD is a one-step process for which there are a wide range of applicable materials; however, its limits include low efficiency, the potential for remaining solvent in the dosage forms and high requirements for solution properties. 6
6
Challenges facing printed pharmaceuticals
Despite the potential of 3D-printed technologies to advance the pharmaceutical industry, the complications impacting application are four-fold: the requirements for excipients, the development of printing software and instrumentation, optimising the mechanical properties of products and the regulatory landscape.
Generally, excipients for use in pharmaceutical 3D printing are relatively limited, compared to traditional manufacturing processes, especially for specialised dosage forms and technologies that use heat. To promote its application by pharma, further investigation into non-toxic, biodegradable, biocompatible and stable excipients is required.6
As the complexity of the structure of a dosage form increases, the modelling and slicing software used to design and inform its production must be continuously updated. The mechanical equipment, operating procedures and control system must also be updated and optimised to meet the needs of the various processes, whether to prevent clogging or promote product uniformity. 6 At present, 3D printers used in pharmaceutical formulation preparation do not meet good manufacturing practice (GMP) standard and thus need to be validated to ensure they meet the required safety standards.
6 At present, 3D printers used in pharmaceutical formulation preparation do not meet good manufacturing practice (GMP) standard and thus need to be validated to ensure they meet the required safety standards.
The mechanical properties of the dosage forms are a quality control parameter – that ensures the products are reproducible and suitable for post-processing. There are various factors that can influence product properties with 3D manufacturing, from nozzle fineness and adhesive viscosity to drying methods and temperature. To ensure the mechanical properties are suitable, the equipment and control programmes must be enhanced, adhesive nozzles refined and printing process parameters optimised.6
In terms of regulation, there are many questions surrounding how 3D-printed pharmaceuticals can be monitored and evaluated for quality. The FDA issued its final guidance on technical considerations for the regulation of 3D-printed medical devices in 2017;7 however, they may not apply to all 3D-printed medical devices as a separate assessment of safety and effectiveness may be required, especially for personalised products. In instances where products are customised to the patient, the question of whether 3D printing is classed as a manufacturing process or compounding, would also impact regulatory guidance. Additionally, though the FDA authorised the first 3D-printed tablets, no regulations or guidelines regarding 3D-printed medicines are currently available. There remain several regulatory challenges, such as how the performance of 3D-printed pharmaceuticals should be measured or their quality controlled, though the FDA’s Office of Testing and Research is currently working to answer them.8
In instances where products are customised to the patient, the question of whether 3D printing is classed as a manufacturing process or compounding, would also impact regulatory guidance. Additionally, though the FDA authorised the first 3D-printed tablets, no regulations or guidelines regarding 3D-printed medicines are currently available. There remain several regulatory challenges, such as how the performance of 3D-printed pharmaceuticals should be measured or their quality controlled, though the FDA’s Office of Testing and Research is currently working to answer them.8
A developing sector
Merck is one of the major players in the pharmaceutical 3D printing sphere, with work undergoing at its Innovation Center to test various technologies, including powder jetting, material extrusion and SLS. In a presentation at PHARMAP 2021, I listened as Dr Christoph Huels, Founder Additive Manufacturing of Tablets at Merck, explained that they are primarily focused on SLS, having found that it creates oral solid dosage forms with desirable mass, hardness, roughness and shape, as well as tablets with uniform API content and comparable dissolution to traditional manufacturing. 9
9
Merck’s current goal, according to Huels, is to establish 3D printing for clinical trial supply. It plans to offer the technology to customers for exploratory studies in the course of the next year while working to develop a GMP solution for market introduction in two or three years’ time. He explained that he anticipates it will be 10-15 years before additive manufacturing is fully developed for broad application in the production of pharmaceuticals, though added that experts do not believe the scale up from the thousands of tablets required for clinical trial phases to commercial scale should be too challenging.9
In a recent interview with 3D Printing Industry,10 Huels stated: “Commercial manufacturing requires so-called ‘blockbuster drugs’ and billions of tablets per year for global supply. 3D printing technologies are not able to secure the supply with the current throughput today. Significant improvements need to be done to reach that in the longer-term future. Nevertheless, the current throughput is already, or will be soon, well suited to supply orphan or smaller oncology indications, where only millions of tablets are required per year.” He added that smaller indications are where 3D printing will likely have the most commercial viability, especially in the short term.
Nevertheless, the current throughput is already, or will be soon, well suited to supply orphan or smaller oncology indications, where only millions of tablets are required per year.” He added that smaller indications are where 3D printing will likely have the most commercial viability, especially in the short term.
Other companies working on the 3D printing of pharmaceuticals include Aprecia, the developer of Spritam – the first 3D-printed drug ever to be approved, and FabRx. Aprecia’s Kirk Donaldson, Vice President Business Development & Alliance Management, recently stated that the company is working to develop new 3D printing formulation platforms, aside from its existing ZipDose and ZipCup binder jetting technologies; the first he said is already clinic and commercial ready.10 Donaldson added that they have several 3D-printed pipeline candidates that will advance to clinic in the next few years.
FabRx launched what it claims is the first GMP-ready 3D printer for pharmaceuticals in 2020,11 the M3DIMAKER, which is currently available for research purposes. “The M3DIMAKER is already taking part in national and international collaborations, which will involve future clinical trials, and we are in communication with the MHRA to aid regulation development for this state-of-the-art technology,” stated the company’s Co-Founder and Director, Alvaro Goyanes. “It combines multiple 3D printing technologies into one machine, allowing for flexible use and increased personalisation potential.”10
“The M3DIMAKER is already taking part in national and international collaborations, which will involve future clinical trials, and we are in communication with the MHRA to aid regulation development for this state-of-the-art technology,” stated the company’s Co-Founder and Director, Alvaro Goyanes. “It combines multiple 3D printing technologies into one machine, allowing for flexible use and increased personalisation potential.”10
Overall, 3D printing has the potential to revolutionise the production of pharmaceutical products, allowing for decentralised and customised manufacturing of therapeutics. However, we will have to wait and see whether the challenges facing the market can be overcome to allow the technology to reach its full therapeutic potential.
References
- Seoane-Viaño I, Trenfield S, Basit A, Goyanes A. Translating 3D printed pharmaceuticals: From hype to real-world clinical applications. Advanced Drug Delivery Reviews. 2021;174:553-575.

- Szczerba R. FDA Approves First 3-D Printed Drug [Internet]. Forbes. 2021 [cited 1 September 2021]. Available from: https://www.forbes.com/sites/robertszczerba/2015/…
- Gioumouxouzis C, Karavasili C, Fatouros D. Recent advances in pharmaceutical dosage forms and devices using additive manufacturing technologies. Drug Discovery Today. 2019;24(2):636-643.
- 3D Printed Drugs Market Research Report by Technology, by Region – Global Forecast to 2025 – Cumulative Impact of COVID-19 [Internet]. Researchandmarkets.com. 2021 [cited 1 September 2021]. Available from: https://www.researchandmarkets.com/reports/4857884/…
- Overview of Pharmaceutical 3D printing | Pharma Excipients [Internet]. Pharma Excipients. 2021 [cited 1 September 2021]. Available from: https://www.pharmaexcipients.com/pharmaceutical-3dp-overview/
- Cui M, Pan H, Su Y, et al. Opportunities and challenges of three-dimensional printing technology in pharmaceutical formulation development.
 Acta Pharmaceutica Sinica B. 2021;11(8):2488-2504.
Acta Pharmaceutica Sinica B. 2021;11(8):2488-2504. - Technical Considerations for Additive Manufactured Medical Devices [Internet]. U.S. Food and Drug Administration. 2017 [cited 1 September 2021]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/…
- Zidan A. Promise and Potential of 3D Printed Pharmaceuticals [Internet]. U.S. Food and Drug Administration. 2017 [cited 1 September 2021]. Available from: https://www.fda.gov/drugs/news-events-human-drugs/cder-researchers-explore-promise-and-potential-3d-printed-pharmaceuticals
- Huels C. 3D printing or Additive Manufacturing of Tablets. Presentation presented at PHARMAP 2021 2021; Virtual.
- Everett H. Merck, Aprecia, and FabRx on transitioning 3D printed pharmaceuticals from lab to clinic – 3D Printing Industry [Internet]. 3D Printing Industry. 2021 [cited 1 September 2021]. Available from: https://3dprintingindustry.com/news/merck-aprecia-and-fabrx…
- FabRx’s pharmaceutical 3D printer for personalised medicines, M3DIMAKER™, is now available! – FabRx [Internet].
 FabRx. 2020 [cited 1 September 2021]. Available from: https://www.fabrx.co.uk/2020/04/06/fabrxs-pharmaceutical-3d-printer-for-personalised-medicines…
FabRx. 2020 [cited 1 September 2021]. Available from: https://www.fabrx.co.uk/2020/04/06/fabrxs-pharmaceutical-3d-printer-for-personalised-medicines…
Related topics
3D printing, Active Pharmaceutical Ingredient (API), Big Pharma, Drug Development, Drug Manufacturing, Formulation, Regulation & Legislation, Research & Development (R&D), Technology, Therapeutics
Related diseases & conditions
Cancer
Five companies personalizing treatments with 3D printed drugs
There has been a noticeable shift in recent years towards the personalization of medicine, with the pharmaceutical industry showing interest in pills that are tailored to a patient’s needs. Here are five companies that are leveraging 3D printing to produce customizable drugs.
Conventional drug production relies on large-scale batch manufacturing, which is very efficient at churning out large volumes of drugs with uniform characteristics. But this becomes time-consuming and expensive when small batches are needed, such as for clinical trials where the doses need to be adjusted. The process is also associated with an environmental burden resulting from inefficient use of raw materials, expired stock and production of toxic and non-toxic waste.
The process is also associated with an environmental burden resulting from inefficient use of raw materials, expired stock and production of toxic and non-toxic waste.
Additive manufacturing, or 3D printing, is a tool that can allow for small-scale production of medicines. This approach uses 3D modeling software to build an object layer-by-layer; for solid drugs, the 3D printer stacks ink with an excipient — inactive substances in drugs added for specific functions like improved stability and bioavailability — and the active pharmaceutical ingredient to print out a finished product. By adjusting the printing parameters, the drug dosages, drug combinations, release profiles and even flavors can be personalized.
There are many challenges that have limited widespread implementation of 3D printing technology, such as 3D printers not meeting good manufacturing practice standards and a limited range of excipients suitable for 3D printing. Nevertheless, 3D printing has the potential to revolutionize the pharmaceutical industry, and several companies are making strides in the space.
Headquarters: Pennsylvania, U.S.
Aprecia Pharmaceuticals is behind the first and only U.S. Food and Drug Administration (FDA)-validated 3D printing platform for commercial-scale drug development. Their aim is to make medicines easier to take and reduce the number of pills a patient needs. Using this platform, Aprecia developed ZipDose Technology, which allows tablets to hold a high dosage load of up to 1,000 mg and still rapidly dissolve with just a sip of water.
Content continues below
Related Content
ZipDose is used in the production of Spritam (levetiracetam), a fast dissolving oral medicine for the treatment of specific types of epileptic seizures. It is targeted for patients who struggle to swallow pills or are unable to measure out the precise dosage of liquid levetiracetam. In 2015, Spritam became the first and only 3D printed drug to be approved by the FDA.
FabRxHeadquarters: London, U. K.
K.
Founded in 2014 as a spin-out of University College London, FabRx aims to revolutionize medical treatments by facilitating the production of personalized medicines, where the size, shape, dosage and dosage combinations can be customized. In 2020, the company released M3DIMAKER, the first pharmaceutical 3D printer for personalized medicine, allowing clinicians and other professionals to “print” tailored medicines on demand.
Using their 3D printing technology, FabRx has also created a range of personalized Printlets — medicines with customized shapes, flavors, colors, dosages and release profiles, such as tablets with Braille and moon patterns for patients with visual impairments as well as Polypills, which combine several drugs in one pill.
FabRx technology is also being used to print personalized pills for children with a rare metabolic disorder called maple syrup urine disease, which requires treatments to be strictly tailored based on age, weight and blood concentrations of the amino acid isoleucine. Current manual preparation methods are time consuming and costly, so FabRx’s approach could save time and money for caregivers.
Current manual preparation methods are time consuming and costly, so FabRx’s approach could save time and money for caregivers.
Headquarters: Darmstadt, Germany
Content continues below
Related Content
The biopharma giant Merck is also taking a stab at next-generation tablet manufacturing through 3D printing. In 2020, Merck announced a cooperation with ACMC — sister company of EOS, a worldwide provider of 3D printing solutions — to produce 3D printed tablets for clinical trials at first, before moving towards commercial-scale manufacturing. This partnership aims to advance the industrial applications of 3D printing technology and digitalization.
Triastek, Inc.Headquarters: Nanjing, China
Triastek is a global leader in pharmaceutical 3D printing, with 41 patents that account for more than 20% of global 3D printing pharmaceuticals applications. Using their proprietary MED 3D printing technology, Triastek develops their own in-house drugs and partners with others to provide drug development solutions.
The MED 3D printing platform allows for the customizable design of tablets, with the different shapes and geometries controlling drug onset time, duration, and drug interactions with the body. This platform was accepted into the FDA’s Emerging Technology Program in 2020, and two of their 3D printed drugs have been granted investigational new drug clearances from the FDA. The first is a chronotherapeutic drug for rheumatoid arthritis, where the drug is taken at bedtime but its release is delayed until the early morning hours, when pain and joint stiffness are at their peak. The second is a one-dose pill alternative to the twice-daily marketed drug for cardiovascular and clotting disorders.
Triastek recently entered into a research collaboration with Eli Lilly and Company to improve the bioavailability of drugs in the intestine by achieving precise organ targeting and programmed drug release.
GlaxoSmithKlineHeadquarters: Brentford, U.K.
GSK was one of the first big pharma companies to show interest in 3D printing as a manufacturing tool, partnering with the University of Nottingham to study the feasibility of 3D inkjet printing and curing with ultraviolet light to produce solid drug forms; in 2017, researchers demonstrated the successful 3D printing of ropinirole tablets for Parkinson’s disease.
3D inkjet printing deposits liquid materials or solid suspensions layer-by-layer but each deposited layer must first be dried, or “cured,” between the deposition steps. GSK is exploring how to convert active pharmaceutical ingredients into curable ink to enable 3D drug printing.
Pharmacy 3D printing | Remedium.ru
Magazine "Remedium" №9, 2020
DOI: 10.21518/1561-5936-2020-9-58-60
Yulia Prozherina, PhD,
REM Analytics
3D printing of medicines is an innovative and economical technology that is an important step towards personalized medicine. This direction can be used to develop drugs with controlled release of active substances; preparations containing combinations with fixed doses, as well as for the creation of orodispersible dosage forms. The global 3D drug market is still largely at the research stage, but is expected to grow rapidly in the next decade [1]. nine0005
3D printing in pharmacy
Yuliya Prozherina, Cand. of Sci. (Bio.),
of Sci. (Bio.),
RM Analytics
3D printing of drugs is an innovative and cost-effective technology, which is a major step towards personalized medicine. This technology can be used for the development of controlled-release drugs; fixed-dose combination drugs, as well as for the creation of orodispersible dosage forms. The global 3D drug market is still largely at the research stage, but its rapid growth is expected in the coming decade [1]. nine0005
FROM CARS TO DRUGS
3D printing was developed in the late 1980s at the Massachusetts Institute of Technology (MIT) as a rapid prototyping method. Currently, it has become widespread and is actively used in industries such as automotive, healthcare (primarily in dentistry and orthopedics) and retail.
Important steps are also being taken towards the development and implementation of 3D medicines into clinical practice. By the way, issued by Therics, Inc. (Princeton, New Jersey) at 19The '94 license covers the use of 3D printing for the production of various products, including medicines (MPs). The application of these technologies in the field of drug delivery has been actively investigated, and in 2015 it was implemented in the United States. In August 2015, the first 3D-printed and FDA-approved LP was produced on an industrial scale. It was the orodispersible antiepileptic drug Spritam (levetiracetam) from Aprecia Pharmaceuticals. The creation of this drug laid the foundation for the 3D future in pharmaceuticals, demonstrating the possibilities of 3D printing for the production of complex dosage forms. Research and development in this area is still ongoing [2]. nine0005
The application of these technologies in the field of drug delivery has been actively investigated, and in 2015 it was implemented in the United States. In August 2015, the first 3D-printed and FDA-approved LP was produced on an industrial scale. It was the orodispersible antiepileptic drug Spritam (levetiracetam) from Aprecia Pharmaceuticals. The creation of this drug laid the foundation for the 3D future in pharmaceuticals, demonstrating the possibilities of 3D printing for the production of complex dosage forms. Research and development in this area is still ongoing [2]. nine0005
IMPORTANT ADVANTAGES
3D drug printing technology becomes an important step in the development of personalized medicine, as it allows implementing the principle of individual selection of components and their dosage depending on the needs of the patient. In addition, when creating a dosage form (DF) according to the presented technology, it is possible to adjust the release profile of the active ingredient depending on the individual characteristics of the patient. Particularly relevant is the creation on the basis of this technology of easily and quickly soluble orodispersible drugs in the oral cavity. nine0005
Particularly relevant is the creation on the basis of this technology of easily and quickly soluble orodispersible drugs in the oral cavity. nine0005
3D printing of drugs may also be in demand in the orphan drug segment, since these drugs are produced in small quantities.
From a technological point of view, the advantages of additive methods in drug development are the ability to accurately control the spatial distribution of pharmaceutical substances, create complex geometries, precipitate small amounts of pharmaceutical substances, reduce waste, and speed up the production of various compositions for screening studies or the manufacture of individualized drugs. The manufacturing benefits associated with LP printing are moving away from traditionally complex, slow and costly production chains and enabling more personalized products without the need for high volume production [2]. To some extent, 3D printing can become a continuation of the pharmacy tradition of manufacturing medicines based on individual recipes, but at a technologically new level and implemented in a slightly different way.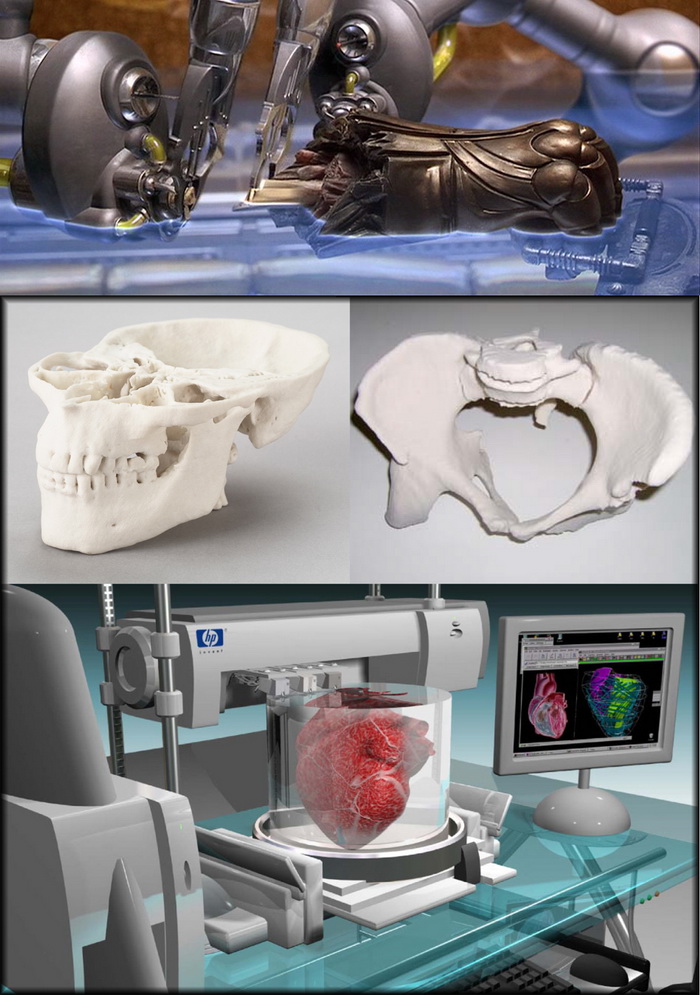 nine0005
nine0005
WORLDWIDE 3D
According to Maximize Market Research, the global 3D medicine market reached $245M in 2019 and is expected to grow to $456M by 2027 (CAGR 8 .07%).
The key drivers of market growth are innovations in 3D printing technologies, as well as an increase in diseases such as epilepsy, schizophrenia, for which the use of instantly soluble dosage forms in the oral cavity is important. On the other hand, unforeseen technological difficulties, the need for large investments in further research, the introduction of various regulation scenarios for this industry segment, as well as the likelihood of side effects of drugs manufactured by this method can hinder further market growth [1, 3]. nine0005
The global 3D printed medicines market can be segmented by dosage form, manufacturing technology and geography.
Based on dosage form, the market is categorized into tablets, capsules, multi-drug implant, nanoparticles, solutions, nanosuspensions, encapsulated polymer and implant [1].
In terms of applied 3D drug manufacturing technology, the market is divided into inkjet printing, direct-write, zip dose, thermal inkjet (TIJ), deposition modeling (FDM), powder printing and stereolithography (SLA) [1]. Despite the fact that over the past 15 years a large number of different 3D printing technologies have been introduced into the LP prototyping industry, inkjet printing is still the most popular [2]. nine0005
Geographically, the market is divided into North America, Europe, Asia Pacific (APAC), South America, and the Middle East and Africa (MEA). The Asia-Pacific region holds the leading market share in the 3D printed medicine market, accounting for 34.1%. It is expected to act as a further growth driver for the segment (CAGR 18.2%). At the same time, countries in the Asia-Pacific region, such as China and India, will grow at the highest rates thanks to huge investments in both R&D and the pharmaceutical industry. In the European region, growth in the development of this technology is also predicted [3]. nine0005
nine0005
Drawing. Global 3D Printed Medicines Market, by Region: 2020-2027
Source: Maximize. Market Research PVT. LTD [3]
There are still few players in the global market for 3D printed medicines. The key ones are GlaxoSmithKline PLC and Aprecia Pharmaceuticals LLC, Fabrx Ltd [1]. Merck, Hewlett Packard Caribe, BV, LLC, 3D Printer Drug Machine, Cycle Pharmaceuticals, etc. are actively engaged in development in this direction [4]. In order to stay in the competitive market, the leading players use various strategies such as acquisitions, mergers, expansions, joint ventures and products. For example, GlaxoSmithKline PLC (GSK) has been investing in 3D printing technology for several years. The R&D department of the 3D printing company uses it in a variety of ways, from drug prototyping to drug packaging. The goal of Fabrx Ltd and GSK is to develop approaches to individualized medicine for each patient using 3D printing [1]. nine0005
Work on the creation of LP using 3D printing technology is also underway in our country. For example, scientists at the St. Petersburg Chemical and Pharmaceutical University plan to create a technology for individual drug dosages using 3D printing. The university has a Russian-made Picasso 3D printer. Another promising project in the field of bioprinting for the university is the creation of drugs with controlled release [5].
For example, scientists at the St. Petersburg Chemical and Pharmaceutical University plan to create a technology for individual drug dosages using 3D printing. The university has a Russian-made Picasso 3D printer. Another promising project in the field of bioprinting for the university is the creation of drugs with controlled release [5].
DRIVER – COVID
While the creation of 3D medicines still requires more research, the production of medical devices and various parapharmaceutical products is progressing more rapidly. In addition, the COVID-19 pandemic has only accelerated this process. So, for example, in many countries of the world this spring, medical products designed to protect against coronavirus were printed on 3D printers. Printed medical masks in the UK were provided by iMark, and in Russia by Temporum (a resident of the Nagatino technopark). Carbon has been actively printing face shields. In Spain, Consorci de la Zona Franca, HP Inc., Leitat and CatSalut have developed the first 3D printed emergency ventilation device. Devices printed on a 3D printer were actively purchased by hospitals in Italy [6]. And this is not the limit of the possibilities of using additive technologies. According to Forbes, the coronavirus pandemic could be a truly high point for 3D printing [7]. nine0005
Devices printed on a 3D printer were actively purchased by hospitals in Italy [6]. And this is not the limit of the possibilities of using additive technologies. According to Forbes, the coronavirus pandemic could be a truly high point for 3D printing [7]. nine0005
References
- 3D Printed Drugs Market Research and Forecast 2018-2027. available at: https://www.omrglobal.com/.
- Blynskaya E.V., Tishkov S.V., Alekseev K.V. 3D printing technologies for the production of dosage forms. Development and registration of medicines . 2018;(24):10–19.
- Global 3D Printed Drugs Market–Industry Analysis and Forecast (2020-2027) – By Scenario, End User and Region. Available at: https://www.maximizemarketresearch.com/. nine0094
- 3D Printed Drugs Market 2019 Top Key Players are 3D Printing Systems, Aprecia Pharmaceuticals, Hewlett Packard Enterprise, Hewlett Packard Enterprise, GlaxoSmithKline PLC and Forecast to 2025. Source: Available at: https://www.
 medgadget.com/.
medgadget.com/. - Using 3D printing technology, it is possible to control drug release in the body. Access mode: https://gxpnews.net/.
- 3D printing in medicine. Access mode: https://zdrav.expert/.
- "Great moment": why the coronavirus pandemic could be the high point for 3D printing. Access mode: https://www.forbes.ru/.
Medical 3D printing | ExCase3D
As the pharmaceutical industry moves away from mass production towards a more personalized model, 3D drug printing could revolutionize the market. The main advantage of using 3D printing technology for personalized pharmaceuticals is the ability to produce small batches with carefully selected dosages, shapes, sizes and characteristics. It also allows flavors to be included in pills without the need for film coating, completely masking the taste of chemical compounds. 3D printers can be installed in pharmacies, hospitals, clinics, etc., making it possible to produce medicines when needed.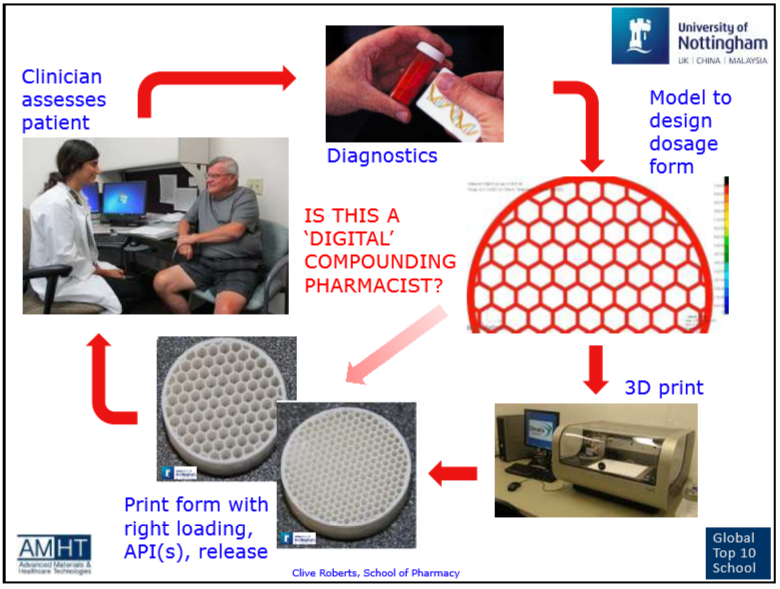 For pharmaceutical companies, 3D printing can significantly reduce costs and waste. nine0005 Aprecia Pharmaceuticals'
For pharmaceutical companies, 3D printing can significantly reduce costs and waste. nine0005 Aprecia Pharmaceuticals'
Spritam (levetiracetam), an antiepileptic drug, is the first and only 3D printed drug. It received FDA approval and was manufactured in 2015 using Aprecia's patented ZipDose technology. The tablet in the mouth completely disintegrates in a few seconds. In addition, very high doses (up to 1000 mg) are achievable, which is usually not possible with conventional drug manufacturing methods. The ZipDose technology uses what is known as drip printing, in which droplets of a liquid binder are applied by a printing nozzle onto a loose layer of pharmaceutical powder, combining the powder into free forms. Then the layer is lowered and another layer of powder is added. Further drops increase the height of the formed tablet. nine0005
In April 2020, the UK-based FabRx launched the first commercially available 3D printer for personalized drug production. M3DIMAKER is an extrusion based printer that allows you to change the printing nozzle according to different dosage requirements./s3/static.nrc.nl/wp-content/uploads/2016/07/ANP-31969064.jpg) Along with the established extrusion-based deposition modeling technology in which the printer melts the pharmaceutical mixture and excipients through a nozzle onto the build plate, the M3DIMAKER also uses another extrusion-based method developed by FabRx: direct powder extrusion (DPE). Similar to Aprecia's ZipDose technology, DPE applies a powdered pharmaceutical agent to the print platform through a nozzle using a single extruder. Both methods allow the production of multi-drug combination pills and sustained or delayed release tablets. nine0005
Along with the established extrusion-based deposition modeling technology in which the printer melts the pharmaceutical mixture and excipients through a nozzle onto the build plate, the M3DIMAKER also uses another extrusion-based method developed by FabRx: direct powder extrusion (DPE). Similar to Aprecia's ZipDose technology, DPE applies a powdered pharmaceutical agent to the print platform through a nozzle using a single extruder. Both methods allow the production of multi-drug combination pills and sustained or delayed release tablets. nine0005
To date, major biopharmaceutical companies have shown minimal activity in this area. In February 2020, Merck KGaA announced a collaboration with AMCM to develop and manufacture 3D printed pharmaceuticals for clinical trials and later for clinical manufacturing. The partnership's stated goal is to develop drugs using nuclear fusion, in which a laser melts and fuses the powder layer by layer. Merck and AMCM expect this will speed up and reduce the cost of tablet production by preventing reformulation.