Meniscus 3d printing
3D Printed Silicone Meniscus Implants: Influence of the 3D Printing Process on Properties of Silicone Implants
. 2020 Sep 18;12(9):2136.
doi: 10.3390/polym12092136.
Eric Luis 1 , Houwen Matthew Pan 2 , Anil Kumar Bastola 1 , Ram Bajpai 3 , Swee Leong Sing 1 , Juha Song 2 , Wai Yee Yeong 1
Affiliations
Affiliations
- 1 Singapore Centre for 3D Printing, School of Mechanical and Aerospace Engineering, Nanyang Technological University, 50 Nanyang Drive, Singapore 639798, Singapore.
- 2 School of Chemical and Biomedical Engineering, Nanyang Technological University, 70 Nanyang Drive, Singapore 639798, Singapore.
- 3 Center for Population Health Sciences, Lee Kong Chien School of Medicine, Nanyang Technological University, 11 Mandalay Road, Singapore 308232, Singapore.
- PMID: 32962059
- PMCID: PMC7570003
- DOI: 10.3390/polym12092136
Free PMC article
Eric Luis et al. Polymers (Basel). .
.
Free PMC article
. 2020 Sep 18;12(9):2136.
doi: 10.3390/polym12092136.
Authors
Eric Luis 1 , Houwen Matthew Pan 2 , Anil Kumar Bastola 1 , Ram Bajpai 3 , Swee Leong Sing 1 , Juha Song 2 , Wai Yee Yeong 1
Affiliations
- 1 Singapore Centre for 3D Printing, School of Mechanical and Aerospace Engineering, Nanyang Technological University, 50 Nanyang Drive, Singapore 639798, Singapore.

- 2 School of Chemical and Biomedical Engineering, Nanyang Technological University, 70 Nanyang Drive, Singapore 639798, Singapore.
- 3 Center for Population Health Sciences, Lee Kong Chien School of Medicine, Nanyang Technological University, 11 Mandalay Road, Singapore 308232, Singapore.
- PMID: 32962059
- PMCID: PMC7570003
- DOI: 10.3390/polym12092136
Abstract
Osteoarthritis of the knee with meniscal pathologies is a severe meniscal pathology suffered by the aging population worldwide. However, conventional meniscal substitutes are not 3D-printable and lack the customizability of 3D printed implants and are not mechanically robust enough for human implantation. Similarly, 3D printed hydrogel scaffolds suffer from drawbacks of being mechanically weak and as a result patients are unable to execute immediate post-surgical weight-bearing ambulation and rehabilitation. To solve this problem, we have developed a 3D silicone meniscus implant which is (1) cytocompatible, (2) resistant to cyclic loading and mechanically similar to native meniscus, and (3) directly 3D printable. The main focus of this study is to determine whether the purity, composition, structure, dimensions and mechanical properties of silicone implants are affected by the use of a custom-made in-house 3D-printer. We have used the phosphate buffer saline (PBS) absorption test, Fourier transform infrared (FTIR) spectroscopy, surface profilometry, thermo-gravimetric analysis (TGA), X-ray photoelectron spectroscopy (XPS), differential scanning calorimetry (DSC), and scanning electron microscopy (SEM) to effectively assess and compare material properties between molded and 3D printed silicone samples.
However, conventional meniscal substitutes are not 3D-printable and lack the customizability of 3D printed implants and are not mechanically robust enough for human implantation. Similarly, 3D printed hydrogel scaffolds suffer from drawbacks of being mechanically weak and as a result patients are unable to execute immediate post-surgical weight-bearing ambulation and rehabilitation. To solve this problem, we have developed a 3D silicone meniscus implant which is (1) cytocompatible, (2) resistant to cyclic loading and mechanically similar to native meniscus, and (3) directly 3D printable. The main focus of this study is to determine whether the purity, composition, structure, dimensions and mechanical properties of silicone implants are affected by the use of a custom-made in-house 3D-printer. We have used the phosphate buffer saline (PBS) absorption test, Fourier transform infrared (FTIR) spectroscopy, surface profilometry, thermo-gravimetric analysis (TGA), X-ray photoelectron spectroscopy (XPS), differential scanning calorimetry (DSC), and scanning electron microscopy (SEM) to effectively assess and compare material properties between molded and 3D printed silicone samples.
Keywords: 3D printing; additive manufacturing; meniscus implants; silicone; validation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Figure 1
The hydrosilylation reaction of liquid…
Figure 1
The hydrosilylation reaction of liquid silicone rubbers.
Figure 1The hydrosilylation reaction of liquid silicone rubbers.
Figure 2
( a ) Photographs: (1)…
Figure 2
( a ) Photographs: (1) motion control platform, (2) discovery extruder, (3) static…
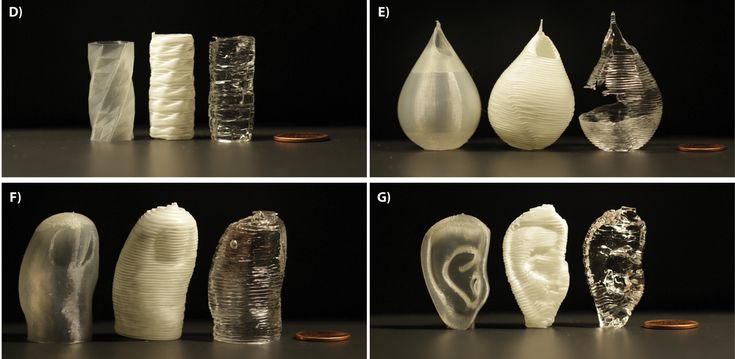
Figure 2(a) Photographs: (1) motion control platform, (2) discovery extruder, (3) static mixer, (4) printer nozzle head, (5) heated printer bed platform, (6) double-barrel-syringe and (b) simplified schematic of experimental setup for heat-cure extrusion-based printer.
Figure 3
Computer-aided design (CAD) of meniscus…
Figure 3
Computer-aided design (CAD) of meniscus implant.
Figure 3Computer-aided design (CAD) of meniscus implant.
Figure 4
Photograph of the standard and…
Figure 4
Photograph of the standard and meniscus samples: ( A ) STD-Eco30 samples (top…
Figure 4Photograph of the standard and meniscus samples: (A) STD-Eco30 samples (top row) and STD-Eco50 samples (bottom row) and (B) 3DP-Eco30 samples (top row) and 3DP-Eco50 samples (bottom row).
Figure 5
Representative stereomicroscopic images of the…
Figure 5
Representative stereomicroscopic images of the core surfaces of silicone meniscus implants. ( a…
Figure 5Representative stereomicroscopic images of the core surfaces of silicone meniscus implants. (a) surface of molded implant, (b) surface of 3D printed implant, (c) cross-section of molded implant and (d) cross-section of 3D printed implant (20× magnification).
Figure 6
Representative scanning electron microscope (SEM)…
Figure 6
Representative scanning electron microscope (SEM) pictures (magnification 100×, 400× and 1000×) showing the…
Figure 6Representative scanning electron microscope (SEM) pictures (magnification 100×, 400× and 1000×) showing the different surface patterns of (a,c,e) molded silicone and (b,d,f) 3D-printed silicone.
Figure 7
Phosphate buffer absorption test for…
Figure 7
Phosphate buffer absorption test for silicone meniscus implants Ecoflex 50 and 30.
Figure 7Phosphate buffer absorption test for silicone meniscus implants Ecoflex 50 and 30.
Figure 8
Surface roughness (µm) of the…
Figure 8
Surface roughness (µm) of the two silicone elastomers (Ecoflex-30 and Ecoflex-50) manufactured by…
Figure 8Surface roughness (µm) of the two silicone elastomers (Ecoflex-30 and Ecoflex-50) manufactured by molding and 3D printing.
Figure 9
FTIR absorbance spectra of (…
Figure 9
FTIR absorbance spectra of ( a ) 3D printed silicone implant and (…
Figure 9FTIR absorbance spectra of (a) 3D printed silicone implant and (b) molded silicone sample.
Figure 10
Thermo-gravimetric analysis/differential scanning calorimentry (TGA/DSC)…
Figure 10
Thermo-gravimetric analysis/differential scanning calorimentry (TGA/DSC) curves of ( a ) molded and (…
Figure 10Thermo-gravimetric analysis/differential scanning calorimentry (TGA/DSC) curves of (a) molded and (b) 3D-Printed silicone Ecoflex 50 measured from 30 to 700 °C at a heat rate of 20 K/min.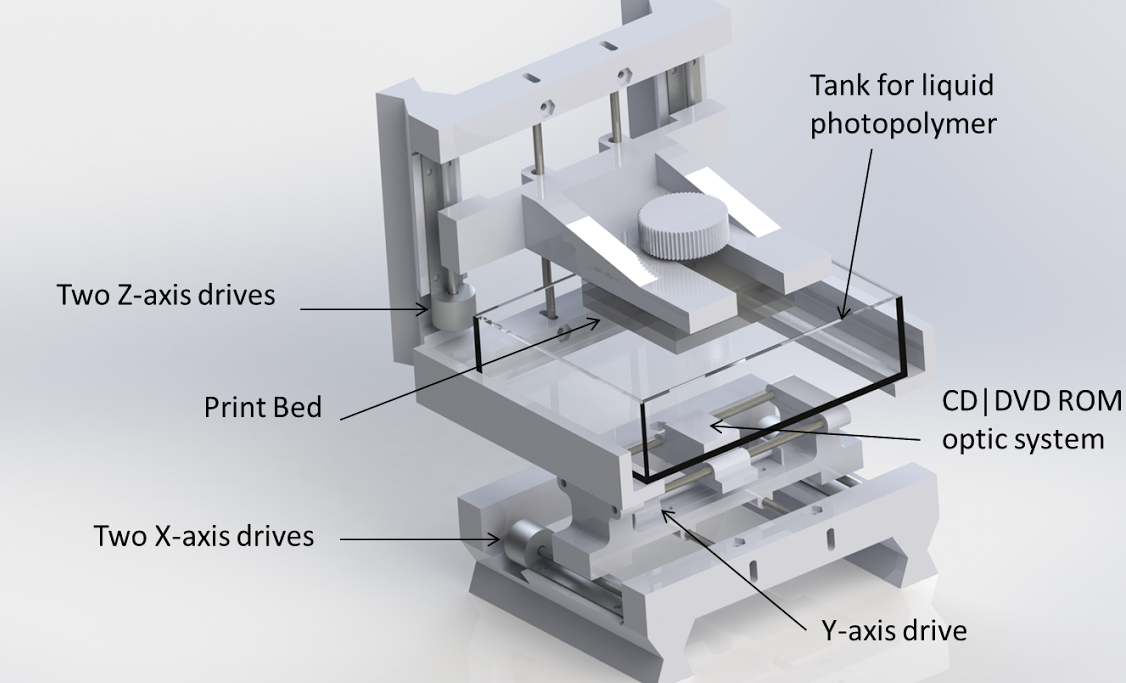 The TGA curve (red) measures the loss of mass and the DSC curve (black) provides information about endothermic and exothermic effects.
The TGA curve (red) measures the loss of mass and the DSC curve (black) provides information about endothermic and exothermic effects.
Figure 10
Thermo-gravimetric analysis/differential scanning calorimentry (TGA/DSC)…
Figure 10
Thermo-gravimetric analysis/differential scanning calorimentry (TGA/DSC) curves of ( a ) molded and (…
Figure 10Thermo-gravimetric analysis/differential scanning calorimentry (TGA/DSC) curves of (a) molded and (b) 3D-Printed silicone Ecoflex 50 measured from 30 to 700 °C at a heat rate of 20 K/min. The TGA curve (red) measures the loss of mass and the DSC curve (black) provides information about endothermic and exothermic effects.
Figure 11
( a ) Cell proliferation…
Figure 11
( a ) Cell proliferation of re-seeded L929 cells on printed/casted Eco50/Eco30 substrates…
Figure 11(a) Cell proliferation of re-seeded L929 cells on printed/casted Eco50/Eco30 substrates after 24, 72, and 120 h culture was quantified based on the WST-8 cell proliferation assay. (b) Fluorescent images of re-seeded L929 cells on printed/casted Eco50/Eco30 substrates after 24, 72, and 120 h culture. Cells were stained with the Live/Dead® cell viability assay. Statistical significance between groups was assessed using two-way analysis of variance (ANOVA) followed by Bonferroni post-tests. ns = p > 0.05 and *** = p < 0.001.
(b) Fluorescent images of re-seeded L929 cells on printed/casted Eco50/Eco30 substrates after 24, 72, and 120 h culture. Cells were stained with the Live/Dead® cell viability assay. Statistical significance between groups was assessed using two-way analysis of variance (ANOVA) followed by Bonferroni post-tests. ns = p > 0.05 and *** = p < 0.001.
Figure 12
Four-cycle cyclic stress-strain for (…
Figure 12
Four-cycle cyclic stress-strain for ( a ) STD-Eco30 and ( b ) STD-Eco50…
Figure 12Four-cycle cyclic stress-strain for (a) STD-Eco30 and (b) STD-Eco50 at strain rates of 12, 120, 360, 720, and 1000 mm/min.
Figure 13
1000-cycle cyclic stress-strain for (…
Figure 13
1000-cycle cyclic stress-strain for ( a ) STD-Eco30 and ( b ) STD-Eco50,…
Figure 131000-cycle cyclic stress-strain for (a) STD-Eco30 and (b) STD-Eco50, at a strain rate of 1000 mm/min.
Figure 14
The 1000-cycle cyclic stress-strain for…
Figure 14
The 1000-cycle cyclic stress-strain for ( a ) 3DP-Eco30 and ( b )…
Figure 14The 1000-cycle cyclic stress-strain for (a) 3DP-Eco30 and (b) 3DP-Eco50, at a strain rate of 1000 mm/min.
Figure 15
Modulus for Eco30 and Eco50…
Figure 15
Modulus for Eco30 and Eco50 at different strain rates.
Figure 15Modulus for Eco30 and Eco50 at different strain rates.
See this image and copyright information in PMC
Similar articles
-
3D Direct Printing of Silicone Meniscus Implant Using a Novel Heat-Cured Extrusion-Based Printer.
Luis E, Pan HM, Sing SL, Bajpai R, Song J, Yeong WY. Luis E, et al. Polymers (Basel). 2020 May 1;12(5):1031. doi: 10.3390/polym12051031. Polymers (Basel). 2020. PMID: 32370046 Free PMC article.
-
3D printed polymeric drug-eluting implants.
Liaskoni A, Wildman RD, Roberts CJ. Liaskoni A, et al. Int J Pharm. 2021 Mar 15;597:120330. doi: 10.1016/j.ijpharm.2021.120330. Epub 2021 Feb 2. Int J Pharm. 2021. PMID: 33540014
-
Three-Dimensional-Printed Scaffolds for Meniscus Tissue Engineering: Opportunity for the Future in the Orthopaedic World.
Vasiliadis AV, Koukoulias N, Katakalos K. Vasiliadis AV, et al. J Funct Biomater. 2021 Dec 2;12(4):69. doi: 10.3390/jfb12040069. J Funct Biomater. 2021. PMID: 34940548 Free PMC article.
-
3D-printing zirconia implants; a dream or a reality? An in-vitro study evaluating the dimensional accuracy, surface topography and mechanical properties of printed zirconia implant and discs.
Osman RB, van der Veen AJ, Huiberts D, Wismeijer D, Alharbi N. Osman RB, et al. J Mech Behav Biomed Mater. 2017 Nov;75:521-528. doi: 10.1016/j.jmbbm.2017.08.018. Epub 2017 Aug 16. J Mech Behav Biomed Mater. 2017. PMID: 28846981
-
3D-printed patient-specific applications in orthopedics.
Wong KC. Wong KC. Orthop Res Rev.
 2016 Oct 14;8:57-66. doi: 10.2147/ORR.S99614. eCollection 2016. Orthop Res Rev. 2016. PMID: 30774470 Free PMC article. Review.
2016 Oct 14;8:57-66. doi: 10.2147/ORR.S99614. eCollection 2016. Orthop Res Rev. 2016. PMID: 30774470 Free PMC article. Review.
See all similar articles
Cited by
-
Recent Progress in Research of Additive Manufacturing for Polymers.
Sing SL, Yeong WY. Sing SL, et al. Polymers (Basel). 2022 Jun 2;14(11):2267. doi: 10.3390/polym14112267. Polymers (Basel). 2022. PMID: 35683939 Free PMC article.
-
A Comprehensive Assessment on the Pivotal Role of Hydrogels in Scaffold-Based Bioprinting.
Parimala Chelvi Ratnamani M, Zhang X, Wang H. Parimala Chelvi Ratnamani M, et al. Gels. 2022 Apr 13;8(4):239. doi: 10.3390/gels8040239. Gels. 2022. PMID: 35448140 Free PMC article.
 Review.
Review. -
Meniscus regeneration by 3D printing technologies: Current advances and future perspectives.
Stocco E, Porzionato A, De Rose E, Barbon S, De Caro R, Macchi V. Stocco E, et al. J Tissue Eng. 2022 Jan 25;13:20417314211065860. doi: 10.1177/20417314211065860. eCollection 2022 Jan-Dec. J Tissue Eng. 2022. PMID: 35096363 Free PMC article. Review.
-
Deposition of Biocompatible Polymers by 3D Printing (FDM) on Titanium Alloy.
Grygier D, Kujawa M, Kowalewski P. Grygier D, et al. Polymers (Basel). 2022 Jan 7;14(2):235. doi: 10.3390/polym14020235. Polymers (Basel). 2022. PMID: 35054641 Free PMC article.
-
Biomaterials and Meniscal Lesions: Current Concepts and Future Perspective.
Lombardo MDM, Mangiavini L, Peretti GM. Lombardo MDM, et al. Pharmaceutics. 2021 Nov 7;13(11):1886. doi: 10.3390/pharmaceutics13111886. Pharmaceutics. 2021. PMID: 34834301 Free PMC article. Review.
See all "Cited by" articles
References
-
- Shane Anderson A., Loeser R.F. Why is osteoarthritis an age-related disease? Best Pract. Res. Clin. Rheumatol. 2010;24:15–26. doi: 10.1016/j.berh.2009.08.006. - DOI - PMC - PubMed
-
- Loeser R.
 F. Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin. Geriatr. Med. 2010;26:371–386. doi: 10.1016/j.cger.2010.03.002. - DOI - PMC - PubMed
F. Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin. Geriatr. Med. 2010;26:371–386. doi: 10.1016/j.cger.2010.03.002. - DOI - PMC - PubMed
- Loeser R.
-
- Jonathan E., Vincenzo C., Claudio Z., Peter V., Ron A., Elliott H., Farshid G., Avi S., Eran L.-G., Emanuele N. A novel polycarbonate-urethane meniscal implant: From bench to first clinical experience. Orthop. Proc. 2012;94-B:125.
-
- Bulgheroni P.
 , Murena L., Ratti C., Bulgheroni E., Ronga M., Cherubino P. Follow-up of collagen meniscus implant patients: Clinical, radiological, and magnetic resonance imaging results at 5 years. Knee. 2010;17:224–229. doi: 10.1016/j.knee.2009.08.011. - DOI - PubMed
, Murena L., Ratti C., Bulgheroni E., Ronga M., Cherubino P. Follow-up of collagen meniscus implant patients: Clinical, radiological, and magnetic resonance imaging results at 5 years. Knee. 2010;17:224–229. doi: 10.1016/j.knee.2009.08.011. - DOI - PubMed
- Bulgheroni P.
-
- Bouyarmane H., Beaufils P., Pujol N., Bellemans J., Roberts S., Spalding T., Zaffagnini S., Marcacci M., Verdonk P., Womack M., et al. Polyurethane scaffold in lateral meniscus segmental defects: Clinical outcomes at 24 months follow-up. Orthop. Traumatol. Surg. Res. 2014;100:153–157. doi: 10.1016/j.otsr.2013.10.011. - DOI - PubMed
Grant support
- Medium-Sized Centre funding scheme/National Research Foundation
- NTU Start-Up Grant/Nanyang Technological University
Meniscus Regenerated with 3-D Printed Implant
Knee Meniscus Regenerated with 3D-Printed Implant
Media files: Image 3.
NEW YORK, NY (December 10, 2014)—Columbia University Medical Center researchers have devised a way to replace the knee’s protective lining, called the meniscus, using a personalized 3-D printed implant, or scaffold, infused with human growth factors that prompt the body to regenerate the lining on its own. The therapy, successfully tested in sheep, could provide the first effective and long-lasting repair of damaged menisci, which occur in millions of Americans each year and can lead to debilitating arthritis. The paper was published today in the online edition of Science Translational Medicine.
“At present, there’s little that orthopedists can do to regenerate a torn knee meniscus,” said study leader Jeremy Mao, DDS, PhD, the Edwin S. Robinson Professor of Dentistry (in Orthopedic Surgery) at the Medical Center. “Some small tears can be sewn back in place, but larger tears have to be surgically removed. While removal helps reduce pain and swelling, it leaves the knee without the natural shock absorber between the femur and tibia, which greatly increases the risk of arthritis.”
Robinson Professor of Dentistry (in Orthopedic Surgery) at the Medical Center. “Some small tears can be sewn back in place, but larger tears have to be surgically removed. While removal helps reduce pain and swelling, it leaves the knee without the natural shock absorber between the femur and tibia, which greatly increases the risk of arthritis.”
A damaged meniscus can be replaced with a meniscal transplant, using tissue from other parts of the body or from cadavers. That procedure, however, has a low success rate and carries significant risks. Approximately one million meniscus surgeries are performed in the United States each year.
Dr. Mao’s approach starts with MRI scans of the intact meniscus in the undamaged knee. The scans are converted into a 3-D image. Data from the image are then used to drive a 3-D printer, which produces a scaffold in the exact shape of the meniscus, down to a resolution of 10 microns (less than the width of a human hair). The scaffold, which takes about 30 minutes to print, is made of polycaprolactone, a biodegradable polymer that is also used to make surgical sutures.
The scaffold is infused with two recombinant human proteins: connective growth factor (CTGF) and transforming growth factor β3 (TGFβ3). Dr. Mao’s team found that sequential delivery of these two proteins attracts existing stem cells from the body and induces them to form meniscal tissue.
For a meniscus to properly form, however, the proteins must be released in specific areas of the scaffold in a specific order. This is accomplished by encapsulating the proteins in two types of slow-dissolving polymeric microspheres, first releasing CTGF (to stimulate production of the outer meniscus) and then TGFβ3 (to stimulate production of the inner meniscus). Finally, the protein-infused scaffold is inserted into the knee. In sheep, the meniscus regenerates in about four to six weeks. Eventually, the scaffold dissolves and is eliminated by the body.
“This is a departure from classic tissue engineering, in which stems cells are harvested from the body, manipulated in the laboratory, and then returned to the patient—an approach that has met with limited success,” said Dr. Mao. “In contrast, we’re jumpstarting the process within the body, using factors that promote endogenous stem cells for tissue regeneration.”
Mao. “In contrast, we’re jumpstarting the process within the body, using factors that promote endogenous stem cells for tissue regeneration.”
"This research, although preliminary, demonstrates the potential for an innovative approach to meniscus regeneration," said co-author Scott Rodeo, MD, sports medicine orthopedic surgeon and researcher at Hospital for Special Surgery in New York City. "This would potentially be applicable to the many patients who undergo meniscus removal each year."
The process was tested in 11 sheep (whose knee closely resembles that of humans). The animals were randomized to have part of their knee meniscus replaced with a protein-infused 3-D scaffold (the treatment group) or a 3-D scaffold without protein (the nontreatment group). After three months, treated animals were walking normally. In a postmortem analysis, the researchers found that the regenerated meniscus in the treatment group had structural and mechanical properties very similar to those of natural meniscus. They are now conducting studies to determine whether the regenerated tissue is long-lasting.
They are now conducting studies to determine whether the regenerated tissue is long-lasting.
“We envision that personalized meniscus scaffolds, from initial MRI to 3-D printing, could be completed within days,” said Dr. Mao. The personalized scaffolds will then be shipped to clinics and hospitals within a week. The researchers hope to begin clinical trials once funding is in place.
“These studies provide clinically valuable information on the use of meniscal regeneration in the knees of patients with torn or degenerate menisci,” said co-author Lisa Ann Fortier, DVM, professor of large animal surgery at Cornell University College of Veterinary Medicine in Ithaca, N.Y. “As a veterinary orthopedic surgeon-scientist on this multi-disciplinary team, I foresee the added bonus of having new techniques for treating veterinary patients with torn knee meniscus.”
3D printing of a knee meniscus scaffold
The article is titled, “Protein-Releasing Polymeric Scaffolds Induce Fibrochondrocytic Differentiation of Endogenous Cells for Knee Meniscus Regeneration in Sheep. ” The other contributors are Chang H. Lee, Chuanyong Lu, and Cevat Erisken, all at CUMC. Scott Rodeo of the Hospital for Special Surgery and Lisa Fortier of Cornell University are two significant collaborators. The authors declare no financial or other conflicts of interest.
The study was funded by grants from the National Institutes of Health (AR065023 and EB009663) jointly to Jeremy Mao, Scott Rodeo, and Lisa Fortier; the Arthroscopy Association of North America; the American Orthopaedic Society for Sports Medicine; and the Harry M. Zweig Foundation.
Columbia University Medical Center provides international leadership in basic, preclinical, and clinical research; medical and health sciences education; and patient care. The medical center trains future leaders and includes the dedicated work of many physicians, scientists, public health professionals, dentists, and nurses at the College of Physicians and Surgeons, the Mailman School of Public Health, the College of Dental Medicine, the School of Nursing, the biomedical departments of the Graduate School of Arts and Sciences, and allied research centers and institutions.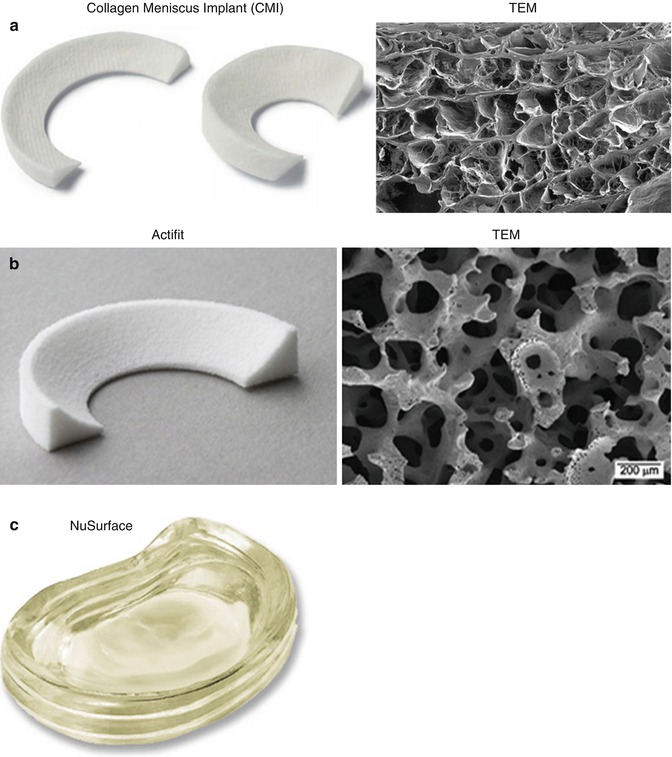 Columbia University Medical Center is home to the largest medical research enterprise in New York City and State and one of the largest faculty medical practices in the Northeast. For more information, visit cumc.columbia.edu or columbiadoctors.org.
Columbia University Medical Center is home to the largest medical research enterprise in New York City and State and one of the largest faculty medical practices in the Northeast. For more information, visit cumc.columbia.edu or columbiadoctors.org.
3D printed knee tissues
This approach works for single cell tissues such as cartilage. Although getting a fully functioning organ and transplanting it into a patient is not easy, researchers at Columbia University Medical Center (CUMC) have succeeded. They used fabrics from the meniscus, the cushioning part of the knee.
A torn meniscus is a very common injury, but at the moment surgeons can only remove the damaged tissue in most cases to relieve pain from irritation and swelling. As a result of such an operation, the knee loses its natural cushioning mechanism, and the risk of arthritis increases. A team of researchers from CUMC, led by Dr. Jeremy Mao, has developed a new technique: a base is created on a 3D printer, the shell of an organ, on which proteins are applied using a high-precision system that stimulate tissue regeneration.
“We believe that a custom-fit meniscus implant can be created in a matter of days, from initial MRI to 3D printing,” says Dr. Mao. Then, within a week, the implants will be sent to hospitals, where they will be transplanted to patients.
Researchers explain that the process of creating an artificial meniscus begins with an MRI scan of a healthy knee, after which the image is converted into a 3D image. This data is used to 3D print an artificial meniscus made from polycaprolactone, a biodegradable polymer. The implants are produced on an EnvisionTEC bioplotter capable of 3D printing with different materials at different temperatures. This process is very similar to the fusion deposition modeling technology commonly used in simple 3D printers, but takes place at a microscopic level.
Sounds unlikely, but 3D printing is the easiest part of the process. However, in order to stimulate stem cells to develop into meniscus tissue, two human proteins must be used in a specific order and in specific amounts. To solve this problem, the researchers decided to use "slowly dissolving microspheres" with proteins inside, which, after transplantation of the meniscus, are introduced into its inner and outer part.
To solve this problem, the researchers decided to use "slowly dissolving microspheres" with proteins inside, which, after transplantation of the meniscus, are introduced into its inner and outer part.
Dr. Mao's theoretical and practical achievements in the creation and transplantation of artificial menisci in humans are the result of previous studies carried out on sheep. Scientists working under the guidance of Dr. Mao believe that their approach differs from the classical engineering of living tissues in that in this case the regeneration process starts after an organ transplant. One of the most important factors in this process is a precise system for stimulating cell growth on a 3D printed shell. Thus, already inside the human body there is a transformation of stem cells, a natural process that also occurs during the development of the embryo.
| 3DNews Technologies and IT market. News printers, print servers, scanners, copiers... The new material allows you to print knee... The most interesting in the reviews 05/02/2017 [14:13], Konstantin Khodakovsky Scientists from the American Duke University have created a material that imitates human cartilage and can eventually be used by surgeons for 3D printing of implants to replace damaged parts of the knee joint, individually shaped to the anatomy of each patient. Human knees have a pair of menisci, crescent-shaped cartilages that act as shock absorbers. But over years of stress, these important parts of the joint can wear out, or be severely damaged by one wrong move while playing football or tennis. The result is pain and an increased risk of developing arthritis. Scientists claim their hydrogel-based material is the first to match the strength and elasticity of human cartilage while remaining stable inside the body and allowing it to be used in the 3D printing process. Duke University Associate Professor of Chemistry and published author Benjamin Wiley notes: “We have now made it possible for everyone to print implants that are very similar in medical properties to human cartilage in a fairly simple and relatively inexpensive way.” . It is worth noting that this is indeed an important achievement. The fact is that torn or damaged menisci are very capricious to the process of self-healing. Surgeons often have to partially or completely remove them, and existing implants either do not match the strength and elasticity of the original, or have poor biocompatibility and prevent the healing of the damaged area. Recently, materials called hydrogels, which are very similar in molecular structure to cartilage and are biologically compatible, are increasingly being used as cartilage replacements. |


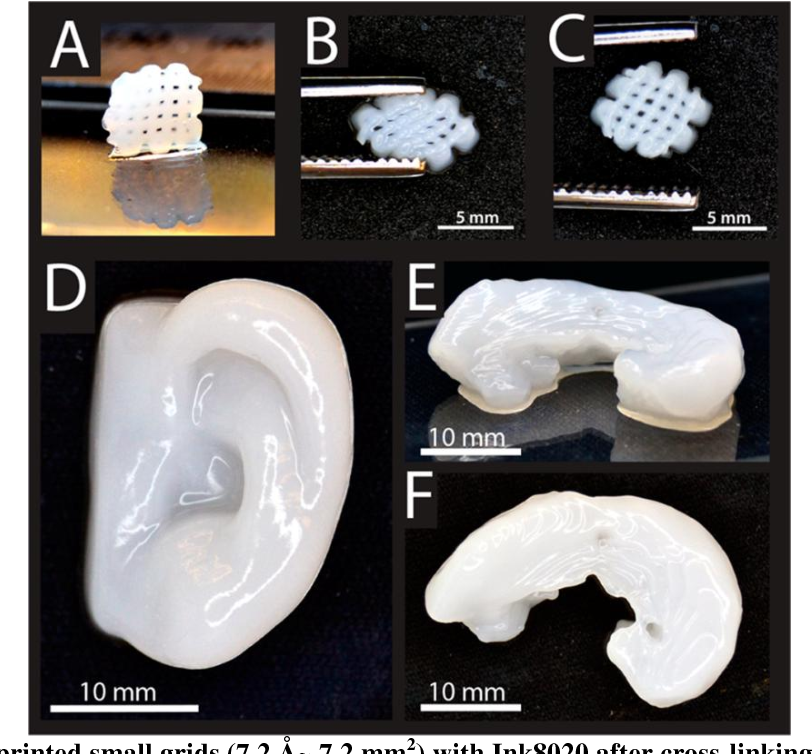 To prove their point, the researchers used a $300 3D printer to print custom menisci for a plastic model of the knee.
To prove their point, the researchers used a $300 3D printer to print custom menisci for a plastic model of the knee.