Google and 3d printing
Bridging Pre-Production Challenges With 3D Printing
The Google Advanced Technology and Projects (ATAP) lab is uniquely set up to be a one-stop-shop for bringing hardware projects to life. The lab offers a vision into the future of both products and production, but uses problem-solving approaches that any company can learn from today, from an iterative mindset at every stage of development, to a technology toolset that enables agile, creative solutions.
In one case, the team’s approach led to a process innovation that allowed them to circumvent a complex supply chain for the pre-production validation stage of an overmolded wearable device. Using Formlabs High Temp Resin, a 3D printing material with high thermal stability, they bridged the gap between prototyping and production, reducing turnaround time for a crucial component by 85% while saving over $100,000.
Watch the video to see how Google ATAP used additive manufacturing to bridge the gap between prototyping and production, using Formlabs 3D printers and High Temp Resin to solve a pre-production challenge for a complex overmolded wearable.
“We're not only investigating what products will look like in the future, but also what production looks like. Additive manufacturing really is a big part of that and ties in very directly with a lot of the projects that we work on,” said Bryan Allen, design technologist at Google ATAP who specializes in 3D printing and advanced fabrication technologies.
“I'm really excited when we can identify something new that's going on in 3D printing, a new material, a new process, and then apply it to a project pipeline in new ways that allow something to be realized more efficiently, faster, better, or more aesthetically pleasing.”
The team was stuck. They were in the pre-production manufacturing phase dialing in the overmolding process for a wearable device, and needed faster results. The process was novel, the supply chain was complex, and they had to get past this in order to ship.
“What we had was electronics that were overmolded, then overmolded again, and that gave us this flexible, waterproof object that we could use in the wearable space,” said David Beardsley, model shop manager for the Google ATAP lab.
Overmolding is a common manufacturing process. For an overmolded part without electronics, a factory might shoot thousands of first articles at a cost of pennies per part or assembly. The ATAP team, however, was doing a second overmold over an overmolded electronic sub-assembly—a printed circuit board assembly (PCBA) with complex electronics that was sourced from another factory—so the first articles were expensive, and reliant on how quickly the first factory could produce and deliver the PCBAs.
Overmolding is an injection molding process that typically requires initial tool tuning at the factory for new parts, which are known as the first articles. These first articles might be underfilled, overfilled, or have cosmetic issues as the molding parameters are calibrated. While troubleshooting, manufacturing engineers ensure that the shutoffs are correct, the pressures are right, and all molding parameters are set correctly.
The wearable device starts as a PCB populated with components. That PCB is encapsulated in a low pressure molding system that turns it into a block of plastic. This PCB and a flexible cable forms the electronic sub-assembly that then gets overmolded in a thermoplastic urethane (TPU) and silicone rubber hybrid. The PCBA then undergoes the final overmolding step.
That PCB is encapsulated in a low pressure molding system that turns it into a block of plastic. This PCB and a flexible cable forms the electronic sub-assembly that then gets overmolded in a thermoplastic urethane (TPU) and silicone rubber hybrid. The PCBA then undergoes the final overmolding step.
The team understood going into pre-production that the cost of first articles would be much higher than for a typical injection molded part. What they had not anticipated was the supply chain bottleneck; it took three weeks to source the overmolded electronic sub-assemblies they needed to run these tests. Beardsley needed to shorten that to ramp up production and ship product.
“You might shoot hundreds and hundreds, thousands of shots, to dial this in. The problem is when you're doing that with live electronics that have real boards that have been stuffed with real electronics and then sent off to the overmolder and then brought back, you've got this whole supply chain,” Beardsley said.
“The light went off when I was watching dollars and weeks just going in the garbage as we were trying to figure out how to dial in the tool. How do we prove this will work before we actually have to put live electronics in there?”
They needed to find a process and material that could stand in for the PCBA. The replacement had to be both dimensionally accurate and represent the exact geometry of the real sub-assembly so that fill could be characterized, and robust enough that the tool could shut off to the part without breaking or deflecting, leading to excessive flash.
“We knew we had to use a material that would withstand thousands of pounds of pressure, north of 250 °C,” Beardsley said. “We were looking for high temperature resistance, high rigidity.”
Beardsley reached out to Allen for help, and they came up with a plan.
“We knew we had to use a material that would withstand thousands of pounds of pressure, north of 250 °C. We were looking for high temperature resistance, high rigidity.
”
David Beardsley
White Paper
Download our white paper for guidelines for using 3D printed molds in the injection molding process to lower costs and lead time and see real-life case studies with Braskem, Holimaker, and Novus Applications.
Read the White Paper
The parameters were tight. Allen decided to try 3D printing the stand-ins, or surrogate parts, in High Temp Resin on the Form 2 stereolithography (SLA) 3D printer. He knew he’d be pushing the boundaries of the material; the final parts would be injected at 270 °C at an injection pressure of 27,000 psi, on the higher end of the published heat deflection temperature (HDT) for High Temp Resin.
Sample part
See and feel Formlabs quality firsthand. We’ll ship a free sample part to your office.
Request a Free Sample Part
“In order to get the small feature size and the shut-offs that we needed, we needed that resolution. It was really the combination of resolution and high temperature resistance that allowed us to use the Form 2 on this,” Allen said. “We have the ability to select from many other fabrication technologies, but having the option to make these parts is really an important piece of our lab.”
It was really the combination of resolution and high temperature resistance that allowed us to use the Form 2 on this,” Allen said. “We have the ability to select from many other fabrication technologies, but having the option to make these parts is really an important piece of our lab.”
“We have the ability to select from many other fabrication technologies, but having the option to make these parts is really an important piece of our lab.”
Bryan Allen
The team quickly got to work, printing some parts to test overnight.
“We had no time to redo any CAD on this. I opened it, exported an STL, and threw it at the PreForm software. Once we got that first batch of validation, we just cranked it. We ran 200 parts the first cycle and then we ran another 100 more,” Allen said.
PreForm is Formlabs’ free print preparation software, which can be used to lay out parts on the build platform in batches. Once they ramped up, Allen printed 250 inserts in batches of 10, which each took about four hours, so the team was able to fabricate hundreds of parts over one weekend.

Allen placed supports on the High Temp parts so that support marks were only on the parts that shut off, not the molding surfaces. This ensured that the parts did not require any additional sanding or finishing beyond a standard wash and post-cure cycle before use.
The 3D printed parts worked perfectly as substitutes for the electronic sub-assemblies. The process reduced the lead time for the PCBA inserts from three weeks to three days, and the cost per insert dropped from $100 to $0.80.
“It allowed us to intercept the process further down the line and save a bunch of upfront steps. Three or four upfront steps were just erased by doing it this way. It saved a bunch of time,” Beardsley said.
David Beardsley shows how the team was able to ‘intercept the process,’ cutting out four production steps, and allowing them to focus on quickly dialing in the final overmolding stage. The 3D printed surrogate parts circumvented the complex supply chain and reduced lead time for insert parts from three weeks to three days.
“The fact that we were able to shut the tool off on 3D printed material, hit it with that high-pressure injection, and not even have it flash, that's a bit unique. Had we not had the Form 2, we would not have been able to pull this off.”
David Beardsley
Because the printed parts were incredibly affordable to produce, the team was able to provide more than the factory had estimated they would need, ensuring they could run shots uninterrupted until they saw satisfactory results.
“The fact that we were able to shut the tool off on 3D printed material, hit it with that high-pressure injection, and not even have it flash, that's a bit unique. Had we not had the Form 2, we would not have been able to pull this off,” Beardsley said.
“When we did move to a full product cycle, we were sure that it was going to work,” Allen said.
Quick look: What did Google ATAP achieve? Using Formlabs Form 2 SLA 3D printer and High Temp Resin, the team was able to:
-
3D print surrogate inserts using High Temp Resin that withstood overmolding with TPU injected at over 250 °C and 27,000 psi.
-
Save an estimated $100,000 in wasted electronic sub-assemblies—even more when accounting for labor costs.
-
Circumvent a complex supply chain to shorten the pre-production validation test cycle for the PCBA inserts from 3 weeks to 3 days.
“When we did move to a full product cycle, we were sure that it was going to work.”
Bryan Allen
While the production process for the wearable device in this case study was unique, the way the team approached the problem, used technology to solve it, and think about 3D printing can be valuable for companies of all size. Find inspiration in these three takeaways from our talk with Google ATAP design technologist Bryan Allen and model shop manager David Beardsley.
Approach every stage of development, from prototyping to shipping the product, with an iterative mindset.
“One of the really unique things about how we work is that we view the entire production process as a prototyping process, where we're running prototype cycles at every stage of production, rather than saying, ‘Okay, prototyping phase, supply chain phase, product launch phase. ’ It's really applying a design process and iteration throughout the entire process rather than just at the beginning,” Allen said.
’ It's really applying a design process and iteration throughout the entire process rather than just at the beginning,” Allen said.
“By the prototyping that we're doing here on every little step of the way, that's when things come to light and that's when you're able to address them before they become bigger problems down the line,” Beardsley said.
Don’t keep technology siloed; consider each machine as part of your set of problem-solving tools that help you find the best solution for each challenge.
“We don't ever try to replace a whole process, we always try to dig down and say, ‘okay what is this machine actually good at it? What is the one thing that this machine does better than anything else, and how do we apply that?’ Not being so devoted to a single process or a single machine allows us to see the merits across many different materials and many different processes and apply them accordingly,” Allen said.
“We see 3D printing as just another tool in the toolset along with CNC and molding and all the more traditional manufacturing processes.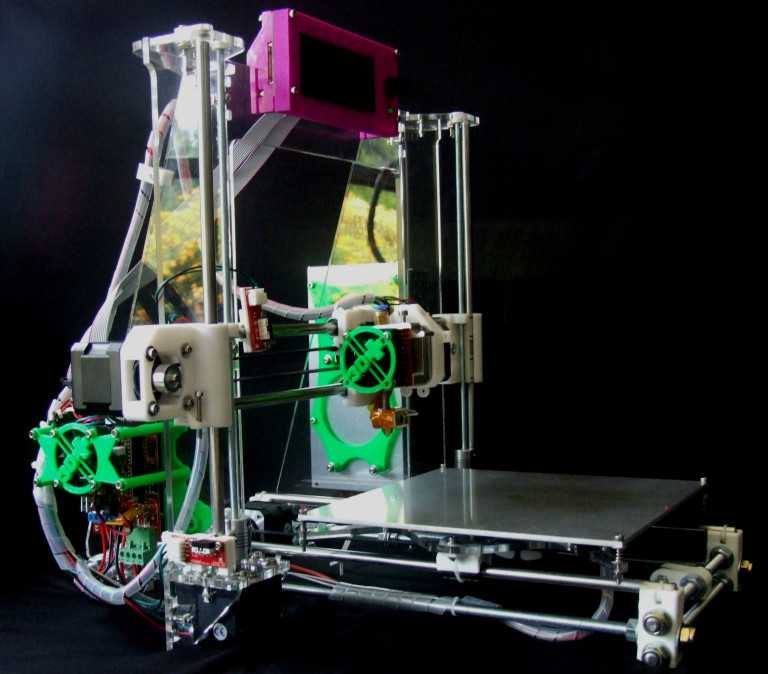 It augments our ability to think about how we apply these new technologies in a production sense.”
It augments our ability to think about how we apply these new technologies in a production sense.”
The Form 2 3D printer is part of the toolset in Google ATAP’s lab that engineers and designers use to solve complex problems, and realize ambitious hardware projects.
Start by solving one problem.
“There's still so many boundaries to the way that parts are designed. For labs that are looking to start applying additive manufacturing into their processes, pick a specific problem that is just one part; rather than trying to print the full tool just try to print an insert; pick one piece and then learn from that,” Allen said.
Learn how a production engineer at Ashley Furniture went from one idea for applying 3D printing to 700 3D printed parts on the factory floor.
The Form 2 and High Temp Resin are part of the variety of tools in the Google ATAP lab that help engineers and designers solve complex problems and realize ambitious hardware projects.
“Any of the big companies that have large shops, they all think, ‘Oh, we've gotta have this really expensive machine.’ We have them here, and they're amazing. The truth is, the Form 2 dominates every day,” Beardsley said. “Accuracy, speed, the finish, the ability to take it right off and have a finished part. It's quite impressive.”
Explore the Form 2 and Formlabs collection of engineering resins for your own project, or request a free sample 3D printed part to see High Temp Resin and other materials firsthand.
Learn more techniques for applying 3D printing in prototyping and production in our Moldmaking With 3D Prints white paper.
Techniques: Moldmaking With 3D Prints
Progressive 3D Printing Technology and Its Application in Medical Materials
Introduction
As an additive manufacturing (AM) technique, three-dimensional (3D) printing enables customized fabrication of 3D constructs based on computer aided design (CAD) software or images obtained from computed tomography (CT) and magnetic resonance imaging (MRI). Firstly developed in the 1980s, 3D printing technology was called rapid prototype technology and has been well applied in a variety of industries with different printing techniques and materials (Liaw and Guvendiren, 2017). With the rapid development of 3D printer, the overall 3D printing market grew to $9.9 billion in 2018 and is expected to reach $34.8 billion in 2024 (MarketsandMarkets, 2019). The medical 3D printing market is expected to maintain significant growth due to the huge potential demand for costumed medical products. Currently, with the expiry of many 3D printing patents (including stereolithography and selective laser sintering), 3D printers and products are becoming cheaper and easy to access (Rahman et al., 2018).
Firstly developed in the 1980s, 3D printing technology was called rapid prototype technology and has been well applied in a variety of industries with different printing techniques and materials (Liaw and Guvendiren, 2017). With the rapid development of 3D printer, the overall 3D printing market grew to $9.9 billion in 2018 and is expected to reach $34.8 billion in 2024 (MarketsandMarkets, 2019). The medical 3D printing market is expected to maintain significant growth due to the huge potential demand for costumed medical products. Currently, with the expiry of many 3D printing patents (including stereolithography and selective laser sintering), 3D printers and products are becoming cheaper and easy to access (Rahman et al., 2018).
3D printing technology has been widely applied in a variety of industries including aviation (Wong, 2016), geoscience (Ishutov et al., 2018), education (Smith and Jones, 2018), clothing (Markstedt et al., 2017), medical (Mitsouras et al., 2015; Giannopoulos et al. , 2016), and pharmaceuticals (Orsi et al., 2015; Norman et al., 2017; Trenfield et al., 2018). Among these medical and pharmaceutical industries, orthopedic and dental applications are favorable to embrace the 3D printing technology (MarketsandMarkets, 2019). It is related to the demand for the patient-specific design and fabrication of the final devices (such as joint prosthesis, surgical guides, and dental restorations) (Eltorai et al., 2015; Tahayeri et al., 2018). Personalized devices manufactured preoperationally are benefited for the efficiency and accuracy (Konta et al., 2017). For medical education and surgical planning, 3D anatomical models are printed subtly with microscopic anatomy structures (Mukherjee et al., 2017; Ganguli et al., 2018). Tissue and organ printing is an emerging field that mainly focused on regenerative medicine and tissue engineering by both academy and industry (Murphy and Atala, 2014). Based on it, preclinical patient-specific disease models are used for drug testings and screenings.
, 2016), and pharmaceuticals (Orsi et al., 2015; Norman et al., 2017; Trenfield et al., 2018). Among these medical and pharmaceutical industries, orthopedic and dental applications are favorable to embrace the 3D printing technology (MarketsandMarkets, 2019). It is related to the demand for the patient-specific design and fabrication of the final devices (such as joint prosthesis, surgical guides, and dental restorations) (Eltorai et al., 2015; Tahayeri et al., 2018). Personalized devices manufactured preoperationally are benefited for the efficiency and accuracy (Konta et al., 2017). For medical education and surgical planning, 3D anatomical models are printed subtly with microscopic anatomy structures (Mukherjee et al., 2017; Ganguli et al., 2018). Tissue and organ printing is an emerging field that mainly focused on regenerative medicine and tissue engineering by both academy and industry (Murphy and Atala, 2014). Based on it, preclinical patient-specific disease models are used for drug testings and screenings. 3D printing technology is merging with traditional pharmaceuticals for the development of dose-customized drugs (Norman et al., 2017).
3D printing technology is merging with traditional pharmaceuticals for the development of dose-customized drugs (Norman et al., 2017).
In this review, recent techniques and applications of 3D printing in medical materials are well summarized. Common AM techniques and printable materials are presented for better understanding of their potential, limitations, and applications. Medical applications including tissue engineering, anatomical models, apparatus, and instruments with 3D printing technology are also provided and summarized. We finally demonstrate our concluding remarks and future outlook on 3D printing in medical materials (Figure 1).
Figure 1 Progressive 3d printing technology and its application in medical materials. Chart showing the application area (yellow boxes) with corresponding products (blue boxes) and primary 3D printing techniques (green boxes).
Current am Technologies and Printable Materials
There are about two dozen AM techniques, among which only some techniques are widely applied in medical industry. The main reason is the specific fabrication process and raw material to meet the high-quality requirements for medical devices. Four common AM techniques are powder-based printing (Brunello et al., 2016), vat polymerization-based printing (Stefaniak et al., 2019), droplet-based printing (Graham et al., 2017), and extrusion-based printing (Taylor et al., 2018).
The main reason is the specific fabrication process and raw material to meet the high-quality requirements for medical devices. Four common AM techniques are powder-based printing (Brunello et al., 2016), vat polymerization-based printing (Stefaniak et al., 2019), droplet-based printing (Graham et al., 2017), and extrusion-based printing (Taylor et al., 2018).
Powder-Based Printing
Powder-based 3D printing is a promising technique with excellent ability for customized fabrication with a variety of external shapes, internal structures, and porosities. There are four common powder-based printing techniques: selective laser sintering (SLS), selective laser melting (SLM), direct metal laser sintering (DMLS), and electron beam melting (EBM) (Figure 2) (Brunello et al., 2016). Every technique is based on localized heating to generate melted metallic powder, which would be used to fabricate the customized products. There are obvious differences in both printing process and product characters among these four powder-based printing techniques. For SLS and DMLS, powder particles are bounded with laser instead of spray solution. In the printing process, the laser draws specific patterns on one layer of the powder bed (Fina et al., 2017). The roller in the printer distributes a new layer of powder onto the surface once the printing of the previous layer is completed. After being built layer-by-layer, printed objects are collected underneath the powder bed. As a specific kind of SLS, DMLS utilizes metal exclusively. Different from sintering techniques, SLM and EBM fully melt powder with laser and electron beam respectively (Wysocki et al., 2018). For the work of electron beam, the powder bed in the EBM printer maintains high working temperature (> 870 K). It directly affects the quality of the fabrication especially in the details of microstructure. Comparatively, products printed with SLM maintain higher tribological, mechanical, and corrosion properties. With the differences between sintering and melting, the surfaces of products printed with sintering techniques (SLS and DMLS) are rough as powders are not completely melted.
For SLS and DMLS, powder particles are bounded with laser instead of spray solution. In the printing process, the laser draws specific patterns on one layer of the powder bed (Fina et al., 2017). The roller in the printer distributes a new layer of powder onto the surface once the printing of the previous layer is completed. After being built layer-by-layer, printed objects are collected underneath the powder bed. As a specific kind of SLS, DMLS utilizes metal exclusively. Different from sintering techniques, SLM and EBM fully melt powder with laser and electron beam respectively (Wysocki et al., 2018). For the work of electron beam, the powder bed in the EBM printer maintains high working temperature (> 870 K). It directly affects the quality of the fabrication especially in the details of microstructure. Comparatively, products printed with SLM maintain higher tribological, mechanical, and corrosion properties. With the differences between sintering and melting, the surfaces of products printed with sintering techniques (SLS and DMLS) are rough as powders are not completely melted.
Figure 2 Printing process of powder-based printing and related products. (A) Schematic diagram. (B) Products manufactured by powder-based printing method. Reproduced, with permission, from (Brunello et al., 2016).
Although the sintering techniques produce products with rough surfaces, they can process with a large variety of materials including plastic powder, ceramic powder, and metal alloys. As the high working temperature, material with volatile constituents (Mg, Zn, Bi, etc.) are not feasible for EBM, while SLM can treat a much wider spectrum of metallic alloys including Ti-based, Al-based, Fe-based, Ni-based, Cu-based, Co-based, and their composites. However, the melting process brings a big advantage that it can produce fully dense parts without post-treatment steps such as infiltration or thermal process.
Vat Polymerization-Based Printing
Vat-polymerization based printing technique is based on light curing resin material and light selective hardening polymerization molding. It is widely used for fabricating complex devices with functional parts such as valves, lenses and fluidic interconnects (Carve and Wlodkowic, 2018). In the process, a vat of photosensitive polymer resin is selectively exposed to a specifically controlled beam of leaser or light (Credi et al., 2016; Credi et al., 2018). The polymer is polymerized after spatially localized irradiation to fabricate the specific constructions (Credi et al., 2016; Wang et al., 2018a). Common process includes digital light processing (DLP), stereolithography (SLA), and multiphoton polymerization (MPP) (Figure 3) (Carve and Wlodkowic, 2018). SLA was the first AM technology applied in medicine in 1994 (Dittmann et al., 1994). A spot laser irradiates the resin localized in a single x-y direction in SLA (Zanchetta et al., 2016), whereas a digital illuminant irradiates the whole x-y plan in DLP (Osman et al., 2017). For both SLA and DLP, the print platform moves parallelly to the z-axis while the final product is fabricated layer-by-layer (Zanchetta et al.
It is widely used for fabricating complex devices with functional parts such as valves, lenses and fluidic interconnects (Carve and Wlodkowic, 2018). In the process, a vat of photosensitive polymer resin is selectively exposed to a specifically controlled beam of leaser or light (Credi et al., 2016; Credi et al., 2018). The polymer is polymerized after spatially localized irradiation to fabricate the specific constructions (Credi et al., 2016; Wang et al., 2018a). Common process includes digital light processing (DLP), stereolithography (SLA), and multiphoton polymerization (MPP) (Figure 3) (Carve and Wlodkowic, 2018). SLA was the first AM technology applied in medicine in 1994 (Dittmann et al., 1994). A spot laser irradiates the resin localized in a single x-y direction in SLA (Zanchetta et al., 2016), whereas a digital illuminant irradiates the whole x-y plan in DLP (Osman et al., 2017). For both SLA and DLP, the print platform moves parallelly to the z-axis while the final product is fabricated layer-by-layer (Zanchetta et al. , 2016; Osman et al., 2017). Differently in MPP, the photosensitive polymer resin is irradiated by a femtosecond laser beam thoroughly in multi directions, resulting that it is not a layer-by-layer technology (Wollhofen et al., 2018). Products printed with vat polymerization technology need to be exposed to light after printing to enhance stability (Credi et al., 2016; Carve and Wlodkowic, 2018).
, 2016; Osman et al., 2017). Differently in MPP, the photosensitive polymer resin is irradiated by a femtosecond laser beam thoroughly in multi directions, resulting that it is not a layer-by-layer technology (Wollhofen et al., 2018). Products printed with vat polymerization technology need to be exposed to light after printing to enhance stability (Credi et al., 2016; Carve and Wlodkowic, 2018).
Figure 3 Polymer scaffold fabricated with SLA approach. (A) Schematic diagram. (B) Products manufactured by vat-polymerization based printing method. Reproduced, with permission, from (Mondschein et al., 2017).
Droplet-Based Printing
Material jetting technology is a process where droplets of liquid materials are ejected and polymerized throughout hundreds of jets. The polymerization only occurs selectively by directed UV for designed structures (Revilla-Leon and Ozcan, 2019). Material jetting technology includes aerosol jet printing (AJP), binder jet printing (BJP), and poly jet printing (PJP) (Figure 4). During AJP, composite in aerosol suspension droplets is carried via N2 gas and ejected onto the substrate layer by layer (Yuan et al., 2017). Multi materials including metals, polymers, and ceramics can be used in AJP with a low printing temperature, which is benefit for biomanufacturing (Mahajan et al., 2013). Binder jet printing (BJP) is similar with SLS except that BJP do not need thermoplastic excipient (Hong et al., 2016). The binder in BJP should meet specific ranges of surface tension (35–40 mJ/N) and viscosity (5–20 Pa·s) (Kim D. H. et al., 2018). In PJP, polymer resin drops are cured by UV light immediately without time consuming postprocessing (Revilla-Leon and Ozcan, 2019). With high resolution, PJP is capable of printing refined structures (Carve and Wlodkowic, 2018).
During AJP, composite in aerosol suspension droplets is carried via N2 gas and ejected onto the substrate layer by layer (Yuan et al., 2017). Multi materials including metals, polymers, and ceramics can be used in AJP with a low printing temperature, which is benefit for biomanufacturing (Mahajan et al., 2013). Binder jet printing (BJP) is similar with SLS except that BJP do not need thermoplastic excipient (Hong et al., 2016). The binder in BJP should meet specific ranges of surface tension (35–40 mJ/N) and viscosity (5–20 Pa·s) (Kim D. H. et al., 2018). In PJP, polymer resin drops are cured by UV light immediately without time consuming postprocessing (Revilla-Leon and Ozcan, 2019). With high resolution, PJP is capable of printing refined structures (Carve and Wlodkowic, 2018).
Figure 4 3D printing of droplet-based printing. (A) Schematic diagram of droplet-based cell printing. (B) Bright-field micrographs of patterned cell junctions containing two cell types. (C) Confocal fluorescence micrographs of cell constructs printed under oil. Reproduced, with permission, from (Brunello et al., 2016).
(C) Confocal fluorescence micrographs of cell constructs printed under oil. Reproduced, with permission, from (Brunello et al., 2016).
Extrusion-Based Printing
Extrusion-based printing was firstly developed by S. Scott Crump in 1988, commonly referred as fused deposition modeling (FDM) or fused filament fabrication (FFF) (Placone and Engler, 2018). FDM is a mature technology based on the extrusion of thermoplastic or composite materials drawn through the hot extrusion head (with one/multiple extrusion nozzles) (Paxton et al., 2017). Fused materials were deposited layer by layer with the horizontal and vertical movement of nozzles controlled by numerically-controlled machine tool (Ozbolat and Hospodiuk, 2016). Extrusion-based printing widely applied in metal printing, polymer printing, and bioprinting (Figure 5) (Ning and Chen, 2017). The printing techniques have been recently developed to precision extrusion deposition (PED) (Fedore et al., 2017), precise extrusion manufacturing (PEM) (Jamroz et al. , 2018), and multiple heads deposition extrusion (MHDS) (Serex et al., 2018). Multiple bioprinting applications in vascular models, soft-tissue models, and bone models manufactured with extrusion-based printing technology have been well-developed in recent years (Ahlfeld et al., 2017; Paxton et al., 2017; Ahlfeld et al., 2018). One major advantage of its bioprinting application is that the hydrogels of extrusion-based printing is capable to fabricate products with high cell density (> 1 × 106 cells ml−1) (Petta et al., 2018; Taylor et al., 2018; Chen et al., 2019).
, 2018), and multiple heads deposition extrusion (MHDS) (Serex et al., 2018). Multiple bioprinting applications in vascular models, soft-tissue models, and bone models manufactured with extrusion-based printing technology have been well-developed in recent years (Ahlfeld et al., 2017; Paxton et al., 2017; Ahlfeld et al., 2018). One major advantage of its bioprinting application is that the hydrogels of extrusion-based printing is capable to fabricate products with high cell density (> 1 × 106 cells ml−1) (Petta et al., 2018; Taylor et al., 2018; Chen et al., 2019).
Figure 5 3D printing of extrusion-based multi-layer printing. (A) Schematic diagram of extrusion-based printing. (B) Multi materials printed with two cell types. (C–F) Available complex organs printed with extrusion-based printing techniques. Reproduced, with permission, from (Placone and Engler, 2018).
Applications in Medical Materials
AM technologies have been widely applied in medical materials, especially in tissue engineering, medical models, medical instruments, and drug formulations. A variety of printing technologies and products have lightened the broad market of medical and chemical applications of 3D printing.
A variety of printing technologies and products have lightened the broad market of medical and chemical applications of 3D printing.
Functional Biomaterials for Tissue Engineering
Tissue engineering with 3D printing has been focused on two parts, functional biomaterials for tissue implantation and tissue models for disease studies. In this section, functional biomaterials manufactured with AM technologies would be the focus. Tissue scaffolds are important component of 3D printing tissue engineering as they can provide structural supports for cell attachment, proliferation and migration (Figure 6). Tissue engineering scaffolds and basic medical scaffolds are considered different especially in biological activity and application purposes (Yang et al., 2018). Good bioactivity, excellent biocompatibility, and appropriate mechanical property are three basic requirements for an ideal tissue engineering scaffold. While basic medical scaffolds are usually applied for filling tissue coloboma or fixation without requirement for bioactivity. Implantable tissue engineering scaffolds are required to be degradable where scaffolds would be replaced by palingenetic tissues (Wang et al., 2018b). To induce tissue or bone growth inside the scaffolds, traditional procedures including molding, freeze drying, and electrospinning have been applied in the manufacture. However, none of the traditional procedures can fabricate scaffolds with customized mechanics, architecture and porosity. With the development of AM, scaffolds with high resolution, customized design, and high porosity have been successful in medical applications.
Implantable tissue engineering scaffolds are required to be degradable where scaffolds would be replaced by palingenetic tissues (Wang et al., 2018b). To induce tissue or bone growth inside the scaffolds, traditional procedures including molding, freeze drying, and electrospinning have been applied in the manufacture. However, none of the traditional procedures can fabricate scaffolds with customized mechanics, architecture and porosity. With the development of AM, scaffolds with high resolution, customized design, and high porosity have been successful in medical applications.
Figure 6 Functional biomaterials and related printing technique. (A) Schematic of a 3D printing platform for performing a water-based biological scaffold. (B) Appearance of 3D printed brackets in various shapes and sizes. Reproduced, with permission, from (Hung et al., 2016).
Tissue engineering scaffolds are fabricated in two major methods, printing with cells mixed in ink or gel and seeding cells onto scaffolds post printing. The most common methods applied in scaffolds fabrication are vat polymerization, SLS, BJ, and FDM. Inkjet printing and extrusion-based printing are the two popular bioprinting technologies, while bio-scaffolds are fabricated based on or without scaffolds. For bioprinting techniques based on scaffolds, hydrogels or polymers laden with cells are cured with AJP, BJP, PJP, and vat polymerization. For bioprinting techniques without scaffolds, hydrogels filled with high cell density (> 1 × 106 cells ml−1) are applied directly relying on cell-cell interactions. Cells in such density need to fuse and mature in the bioreactor for a period of time.
The most common methods applied in scaffolds fabrication are vat polymerization, SLS, BJ, and FDM. Inkjet printing and extrusion-based printing are the two popular bioprinting technologies, while bio-scaffolds are fabricated based on or without scaffolds. For bioprinting techniques based on scaffolds, hydrogels or polymers laden with cells are cured with AJP, BJP, PJP, and vat polymerization. For bioprinting techniques without scaffolds, hydrogels filled with high cell density (> 1 × 106 cells ml−1) are applied directly relying on cell-cell interactions. Cells in such density need to fuse and mature in the bioreactor for a period of time.
Only a few companies have launched commercial tissue engineering scaffolds. Organogenesis Inc, one of the world's most famous FDA-approved 3D printed medical device supplier, introduced their GINTUIT™, a tissue engineering product approved in 2012 for oral soft tissue repair and regeneration. It is a commercialized cell and gene therapy product combined fibroblasts and keratinocytes in bovine collagen. In 2016, another famous supplier, Stryker released the product Tritanium® LP, a titanium lumber posterior cage. The lumber cage is fabricated with abundant porous by DMLS technology with titanium alloy. The inner porous are helpful for blood vessel and bone growth inside the lumber cage.
In 2016, another famous supplier, Stryker released the product Tritanium® LP, a titanium lumber posterior cage. The lumber cage is fabricated with abundant porous by DMLS technology with titanium alloy. The inner porous are helpful for blood vessel and bone growth inside the lumber cage.
With widespread concerns from various industries, bioprinting and tissue engineering have made significant progress and wide applications. Applications covered profuse tissues including tooth, bone, cartilage, ear, blood vessel, liver, kidney, and myocardium (Zhu et al., 2019). In 2017, Monica M. Laronda et al. from Northwestern university claimed successful fabrication of a bioprosthetic ovary created using 3D printed microporous scaffolds restoring ovarian function in sterilized mice (Laronda et al., 2017). Recently, Byoung Soo Kim and his colleges developed human skin with PJP 3D printing system (Ahn et al., 2016). This printed skin showed favorable biological characteristics, including stable dermis and epidermal layers. Manufactured skin substitutes can significantly improve skin healing of the wound area.
Manufactured skin substitutes can significantly improve skin healing of the wound area.
Anatomical and Pharmacological Models
To date, 3D printed tissue models play a significant role in the studies of mechanism of disease, pharmacological testing for new drugs, effectiveness of preclinical therapy, and anatomical structures of complicated organs (Figures 7 and 8). For these studies, conventional methods take plenty of mice and other experiment animals for building animal models. Typically, patient derived xenograft (PDX) models for medical studies always cost a large amount of immunodeficient mice to engraft disease cells. This kind of process takes a great mass of time and money. To overcome the disadvantage, tissue models were developed, firstly by traditional fabrication technologies without 3D printing. However, products by traditional methods revealed inaccurate models with unrealistic tissue status. With the application of 3D printing, biomimetic tissue models with high resolution are fabricated more efficiently at a lower cost than in the past. In this part, 3D printed tissue models of skin, liver, and tumor would be discussed.
In this part, 3D printed tissue models of skin, liver, and tumor would be discussed.
Figure 7 Anatomical 3D models of heart in normal and pathological state. (A) Normal anatomical 3D printed heart model. (B) Tetralogy of Fallot anatomical 3D printed heart model. Reproduced, with permission, from (Bartel et al., 2018).
Figure 8 Applications and limitations of 3D organ models in pharmacological research. Reproduced, with permission, from (Weinhart et al., 2019).
The liver is a complex organ with multiple functions which have biotransformation effects on many non-nutritive substances (such as various drugs, poisons, and certain metabolites) in the body. They are completely decomposed by metabolism or excreted in the original form. With highly sensitive to drug toxicity, liver tissue engineering models were developed to take drug screening and testing. Reproducing the complex structures with 3D printing technology is the basic step for mimicking hepatic functions. To date, multiple fabrications of liver tissue models were accomplished with several 3D printing technologies. Ho-Joon Lee et al. developed multicellular 3D liver with multi-functions encapsulated in hybrid hydrogel (Lee et al., 2017). HepaRG cells alone or with supporting cells were encapsulated in semi-IPNs (hydrogel) and printed with vat polymerization technology. Fabricated 3D liver model was verified to be functional maturation with a dynamic 3D microenvironment, which is important for disease modeling and drug testing. Huanhuan Joyce Chen et al. fabricated a 3D scaffold co-cultured with human intestinal cells (hIECs) and liver cells to mimic a two-organ body-on-chip situation (Chen et al., 2018). The hIECs and liver cells in this scaffold were verified to maintain high viability and differentiable. While hIECs differentiated into human gastrointestinal cells, liver cells developed into lobule-like structures. Two organs on chip 3D model significantly improved the studies on human response and Inter-organ relationships.
To date, multiple fabrications of liver tissue models were accomplished with several 3D printing technologies. Ho-Joon Lee et al. developed multicellular 3D liver with multi-functions encapsulated in hybrid hydrogel (Lee et al., 2017). HepaRG cells alone or with supporting cells were encapsulated in semi-IPNs (hydrogel) and printed with vat polymerization technology. Fabricated 3D liver model was verified to be functional maturation with a dynamic 3D microenvironment, which is important for disease modeling and drug testing. Huanhuan Joyce Chen et al. fabricated a 3D scaffold co-cultured with human intestinal cells (hIECs) and liver cells to mimic a two-organ body-on-chip situation (Chen et al., 2018). The hIECs and liver cells in this scaffold were verified to maintain high viability and differentiable. While hIECs differentiated into human gastrointestinal cells, liver cells developed into lobule-like structures. Two organs on chip 3D model significantly improved the studies on human response and Inter-organ relationships. The two 3D liver models above were well fabricated and suitable for short-term studies. To realize long-term studies with functional liver tissue models, Hassan Rashidi et al. developed a stable 3D liver tissue model with certain function, which is testified for 1 year (Rashidi et al., 2018). Mimicking realistic conditions, hexagonal scaffolds were fabricated with polycaprolactone embraced with self‐aggregated pluripotent stem cells (PSCs) spheroids. Embedding with PSCs-loaded implants, two mice models of tyrosinemia were claimed to heal without any infection. Emerging 3D liver tissue models are helping solving problems in an efficient and cost-effective way that we cannot imagine before.
The two 3D liver models above were well fabricated and suitable for short-term studies. To realize long-term studies with functional liver tissue models, Hassan Rashidi et al. developed a stable 3D liver tissue model with certain function, which is testified for 1 year (Rashidi et al., 2018). Mimicking realistic conditions, hexagonal scaffolds were fabricated with polycaprolactone embraced with self‐aggregated pluripotent stem cells (PSCs) spheroids. Embedding with PSCs-loaded implants, two mice models of tyrosinemia were claimed to heal without any infection. Emerging 3D liver tissue models are helping solving problems in an efficient and cost-effective way that we cannot imagine before.
As one of the largest organs in human body, skin covers the whole-body surface and plays an important role in protecting, excreting, regulating body temperature, and feeling external stimuli. For patients with extensive skin wounds, clinical therapies would be complicated and important. To test the efficacy and safety of treatment, skin tissue models reveal an irreplaceable role. Byoung Soo Kim et al. fabricated a 3D printing skin tissue model with skin-derived extracellular matrix (S-dECM) bioink (Kim B. S. et al., 2018). Embraced in vivo with endothelial progenitor cells (EPCs) and adipose-derived stem cells (ASCs), 3D printing skin model accelerated wound healing especially in reepithelization and neovascularization. John W. Wills et al. adapted nanoparticles in 3D reconstructed skin micronucleus (RSMN) assay (Wills et al., 2016). After normalizing the dose between the total nanoparticle mass and the cell number between 2D/3D assays, the 3D dose response was compared to the 2D micronucleus assay. Due to the protective properties of the 3D cell microstructure and the mixed barrier effect, tested silica particles revealed no (gene) toxicity for live cells in the 3D model comparing to the 2D assay. Plenty results suggested 3D skin model can more accurately reflect the toxicity of nanoparticle drugs on human skin function than traditional methods.
Byoung Soo Kim et al. fabricated a 3D printing skin tissue model with skin-derived extracellular matrix (S-dECM) bioink (Kim B. S. et al., 2018). Embraced in vivo with endothelial progenitor cells (EPCs) and adipose-derived stem cells (ASCs), 3D printing skin model accelerated wound healing especially in reepithelization and neovascularization. John W. Wills et al. adapted nanoparticles in 3D reconstructed skin micronucleus (RSMN) assay (Wills et al., 2016). After normalizing the dose between the total nanoparticle mass and the cell number between 2D/3D assays, the 3D dose response was compared to the 2D micronucleus assay. Due to the protective properties of the 3D cell microstructure and the mixed barrier effect, tested silica particles revealed no (gene) toxicity for live cells in the 3D model comparing to the 2D assay. Plenty results suggested 3D skin model can more accurately reflect the toxicity of nanoparticle drugs on human skin function than traditional methods.
Tumor is a new pathological organism formed by the proliferation of local tissue cells under the action of various tumorigenic factors, and has an extremely complex microenvironment and microstructure. It is significant to mimic in vivo tumor environment with stroma and micro structures for the accuracy of testing new theories and therapies (Costa et al., 2016). Jizhao Li et al. developed a 3D cell model with human lung cancer A549 cells applied in scaffolds fabricated with silk fibroin protein and chitosan (Li et al., 2018). By resembling pathological conditions, the 3D tumor model provide a valuable biomaterial platform for in-vitro test of antitumor drugs for non-small cell lung cancer. Yu Zhao et al. fabricated a 3D in vitro cervical tumor model with 3D printing of Hela cells in hydrogel grid structure by a layer-by-layer fashion (Zhao et al., 2014). With higher proliferation rate and matrix metalloproteinase protein expression, 3D cervical model fabricated with novel 3D printing technology is helping cervical tumor studies. Therefore, with the development of 3D printing tissue models, it is credible for the promising future that studies will be done more efficiently without sacrifices from experimental animals.
It is significant to mimic in vivo tumor environment with stroma and micro structures for the accuracy of testing new theories and therapies (Costa et al., 2016). Jizhao Li et al. developed a 3D cell model with human lung cancer A549 cells applied in scaffolds fabricated with silk fibroin protein and chitosan (Li et al., 2018). By resembling pathological conditions, the 3D tumor model provide a valuable biomaterial platform for in-vitro test of antitumor drugs for non-small cell lung cancer. Yu Zhao et al. fabricated a 3D in vitro cervical tumor model with 3D printing of Hela cells in hydrogel grid structure by a layer-by-layer fashion (Zhao et al., 2014). With higher proliferation rate and matrix metalloproteinase protein expression, 3D cervical model fabricated with novel 3D printing technology is helping cervical tumor studies. Therefore, with the development of 3D printing tissue models, it is credible for the promising future that studies will be done more efficiently without sacrifices from experimental animals.
Medical Apparatus and Instruments
AM is a promising and novel technology for the production of medical apparatus and instruments comparing with traditional manufacturing techniques. Directed by patient's clinical images, custom-designed medical apparatus and surgical guides are fabricated efficiently and accurately. It brings anatomically fit to patients and surgical safety to surgeons. Besides, AM is capable of manufacturing complex microstructures which are not possible for conventional techniques (Figure 9). With these advantages, AM allows fast production with high resolution, few leftover material and low costs. In this section, discussion is focused on (i) prostheses and implants and (Heidari Keshel et al., 2016) auxiliary medical equipment.
Figure 9 Medical apparatus and instruments by 3D printing. (A) 3D printed guide template for surgery simulation. (B, C) 3D printed titanium apparatus for cervical spine and pelvic surgery respectively. Reproduced, with permission, from (Xu et al., 2016; Wei et al., 2017; Nie et al., 2019).
Reproduced, with permission, from (Xu et al., 2016; Wei et al., 2017; Nie et al., 2019).
Medical implants and orthoses/prostheses (O&P) have been fabricated with traditional methods for decades. As with long term application, conservative implants revealed problems including anatomical mismatching, incompetent binding strength and initial stability, poor bone ingrowth and long-term stability, and low cost-efficiency (Wang et al., 2019). All these problems have been solved with AM technology, which is capable to fabricate implants with proper surface and mechanical properties. Powder-based 3D printing techniques (SLM, SLS, and EBM) are widely applied in implants and O&P manufacturing as they are compatible with a wide range of printing materials, such as titanium alloy, zinc alloy, cobalt–chrome alloy, and polyetheretherketone (PEEK). With outstanding mechanical properties and biocompatibility, 3D printed implants have been applied in plenty of surgical majors, including tracheobronchial (Zopf et al. , 2014; Han et al., 2018), dentofacial, cardiovascular, orthopedic, and spine. For severe tracheobronchomalacia patients, a 3D printed self-expandable, metallic tracheobronchial stent fabricated with SLS technology was implanted into patient's collapse bronchus and rebuilt airway efficiently (Han et al., 2018). The printing technology offer a great opportunity of reconstruction and support for tracheobronchial diseases, which was difficult for conservative implants to be fabricated anatomically fitted. Besides self-expandable stent, a 3D printed bioresorbable stent was fabricated with SLS technique (Zopf et al., 2014). Printed bioresorbable stents were embedded into severe tracheobronchomalacia pig model, significant resolution of symptoms was observed. The stent was resorbed over time and was considered as a “4D” functional material. In maxillofacial and craniofacial surgeries, complex anatomy structures and irregular shapes of defects are the two most severe challenges. Conventionally, craniofacial prostheses are fabricated with hand-curved wax model for the anatomic defect with low precision.
, 2014; Han et al., 2018), dentofacial, cardiovascular, orthopedic, and spine. For severe tracheobronchomalacia patients, a 3D printed self-expandable, metallic tracheobronchial stent fabricated with SLS technology was implanted into patient's collapse bronchus and rebuilt airway efficiently (Han et al., 2018). The printing technology offer a great opportunity of reconstruction and support for tracheobronchial diseases, which was difficult for conservative implants to be fabricated anatomically fitted. Besides self-expandable stent, a 3D printed bioresorbable stent was fabricated with SLS technique (Zopf et al., 2014). Printed bioresorbable stents were embedded into severe tracheobronchomalacia pig model, significant resolution of symptoms was observed. The stent was resorbed over time and was considered as a “4D” functional material. In maxillofacial and craniofacial surgeries, complex anatomy structures and irregular shapes of defects are the two most severe challenges. Conventionally, craniofacial prostheses are fabricated with hand-curved wax model for the anatomic defect with low precision. With 3D printing techniques, patient-customized prostheses are fabricated with guidance from CT or MRI images in which details for defects are well recorded. Kyle K et al. demonstrated the first application case of 3D printing in complex fetal craniofacial anomalies and perinatal management (VanKoevering et al., 2015). Researchers from Saint Louis University School of Medicine reviewed 315 patients who underwent 3D printing assisted maxillofacial and craniofacial surgeries (Jacobs and Lin, 2017). Fabrications with 3D printing techniques were mainly focused on contour models, surgical guides, splints, and implants. These objects were mainly fabricated in factory and laboratory with an average time and cost of 18.9 h and $1,353.31 respectively. Without lab or proficiency with printing software, low-cost 3D maxillofacial models could be fabricated with a cost of only $90 (Legocki et al., 2017). While commercial models can be manufactured with serializable materials and advanced virtual planning, this low-cost method can generate models with high-fidelity as educational and surgical planning tools.
With 3D printing techniques, patient-customized prostheses are fabricated with guidance from CT or MRI images in which details for defects are well recorded. Kyle K et al. demonstrated the first application case of 3D printing in complex fetal craniofacial anomalies and perinatal management (VanKoevering et al., 2015). Researchers from Saint Louis University School of Medicine reviewed 315 patients who underwent 3D printing assisted maxillofacial and craniofacial surgeries (Jacobs and Lin, 2017). Fabrications with 3D printing techniques were mainly focused on contour models, surgical guides, splints, and implants. These objects were mainly fabricated in factory and laboratory with an average time and cost of 18.9 h and $1,353.31 respectively. Without lab or proficiency with printing software, low-cost 3D maxillofacial models could be fabricated with a cost of only $90 (Legocki et al., 2017). While commercial models can be manufactured with serializable materials and advanced virtual planning, this low-cost method can generate models with high-fidelity as educational and surgical planning tools. Cardiac diseases have been widely studied with the assistance of 3D printing technology as it offers high-resolution reduction of pathological status (Vukicevic et al., 2017). Variety of printing techniques including material jetting (Olivieri et al., 2015; Lind et al., 2017; Lau et al., 2018; Su et al., 2018), FDM (Mahmood et al., 2015; Son et al., 2015), SLS, and SLA have been applied in the studies of structural heart disease, congenital heart disease, coronary arteries, and systemic vasculature. Benefiting from 3D printing techniques, advanced visualization (Mahmood et al., 2015; Olivieri et al., 2015), diagnosis (Son et al., 2015), planning of surgeries, interventions (Lind et al., 2017), education (Lau et al., 2018; Su et al., 2018), and researches (Mahmood et al., 2015; Lind et al., 2017) in cardiovascular diseases are developing rapidly.
Cardiac diseases have been widely studied with the assistance of 3D printing technology as it offers high-resolution reduction of pathological status (Vukicevic et al., 2017). Variety of printing techniques including material jetting (Olivieri et al., 2015; Lind et al., 2017; Lau et al., 2018; Su et al., 2018), FDM (Mahmood et al., 2015; Son et al., 2015), SLS, and SLA have been applied in the studies of structural heart disease, congenital heart disease, coronary arteries, and systemic vasculature. Benefiting from 3D printing techniques, advanced visualization (Mahmood et al., 2015; Olivieri et al., 2015), diagnosis (Son et al., 2015), planning of surgeries, interventions (Lind et al., 2017), education (Lau et al., 2018; Su et al., 2018), and researches (Mahmood et al., 2015; Lind et al., 2017) in cardiovascular diseases are developing rapidly.
Conclusion
This paper reviews the advancements of 3D printing technologies applied in medical materials in recent years. With the superiority of patient-specific designs, high complexity, favorable productivity, and cost-effective manufacturing methods, 3D printing has been becoming widely accepted manufacturing technologies in the medical applications. The main applications of 3D printing in medicine include tissue engineering models, anatomical models, pharmacological designs and validation models, medical apparatus and instruments. Orthopedics is one of the most advanced fields that integrate 3D printing to produce end-use products such as restorations, spine models, and surgical navigation boards. Orthopedics is a pioneer in medical devices. Currently, there are many multiple 3D printed medical products on the market, including implantable craniofacial implants, acetabular cups, knee implants, spinal cages, and surgical instruments. In addition, about 99% of hearing aid housings are custom made through 3D printing. Pre-surgery printed anatomical models have revolutionized the way surgeons and medical students were trained for surgery. To date, researchers have printed about 16 different types of tissues, providing tissue models for high-throughput screening for new drugs. It is believed that 3D printing is affecting clinical and related basic research in an increasingly broader manner.
The main applications of 3D printing in medicine include tissue engineering models, anatomical models, pharmacological designs and validation models, medical apparatus and instruments. Orthopedics is one of the most advanced fields that integrate 3D printing to produce end-use products such as restorations, spine models, and surgical navigation boards. Orthopedics is a pioneer in medical devices. Currently, there are many multiple 3D printed medical products on the market, including implantable craniofacial implants, acetabular cups, knee implants, spinal cages, and surgical instruments. In addition, about 99% of hearing aid housings are custom made through 3D printing. Pre-surgery printed anatomical models have revolutionized the way surgeons and medical students were trained for surgery. To date, researchers have printed about 16 different types of tissues, providing tissue models for high-throughput screening for new drugs. It is believed that 3D printing is affecting clinical and related basic research in an increasingly broader manner.
Author Contributions
DF and YL contributed equally to this reviewed manuscript. XW, YT and ZL conceived and designed the content of the manuscript. DF, YL and XW collected the researched literatures, arranged the outline of collected documents and wrote the articles. TZ, QW, HC, and WL made important suggestions and helped revising the manuscript. All authors reviewed and commented on the entire manuscript.
Funding
This research was funded by the Ministry of Science and Technology of China (2016YFB1101501 and 2018YFE0104200) and National Natural Science Foundation of China (NSFC, 51973226).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor and reviewer JD declared their involvement as co-editors in the Research Topic, and confirm the absence of any other collaboration.
References
Ahlfeld, T. , Cidonio, G., Kilian, D., Duin, S., Akkineni, A. R., Dawson, J. I., et al. (2017). Development of a clay based bioink for 3D cell printing for skeletal application. Biofabrication 9 (3), 034103. doi: 10.1088/1758-5090/aa7e96
, Cidonio, G., Kilian, D., Duin, S., Akkineni, A. R., Dawson, J. I., et al. (2017). Development of a clay based bioink for 3D cell printing for skeletal application. Biofabrication 9 (3), 034103. doi: 10.1088/1758-5090/aa7e96
PubMed Abstract | CrossRef Full Text | Google Scholar
Ahlfeld, T., Doberenz, F., Kilian, D., Vater, C., Korn, P., Lauer, G., et al. (2018). Bioprinting of mineralized constructs utilizing multichannel plotting of a self-setting calcium phosphate cement and a cell-laden bioink. Biofabrication 10 (4), 045002. doi: 10.1088/1758-5090/aad36d
PubMed Abstract | CrossRef Full Text | Google Scholar
Ahn, S. H., Lee, J., Park, S. A., Kim, W. D. (2016). Three-dimensional bio-printing equipment technologies for tissue engineering and regenerative medicine. Tissue Eng. Regener. Med. 13 (6), 663–676. doi: 10.1007/s13770-016-0148-1
CrossRef Full Text | Google Scholar
Bartel, T., Rivard, A., Jimenez, A., Mestres, C. A., Muller, S. (2018). Medical three-dimensional printing opens up new opportunities in cardiology and cardiac surgery. Eur. Heart J. 39 (15), 1246–1254. doi: 10.1093/eurheartj/ehx016
(2018). Medical three-dimensional printing opens up new opportunities in cardiology and cardiac surgery. Eur. Heart J. 39 (15), 1246–1254. doi: 10.1093/eurheartj/ehx016
PubMed Abstract | CrossRef Full Text | Google Scholar
Brunello, G., Sivolella, S., Meneghello, R., Ferroni, L., Gardin, C., Piattelli, A., et al. (2016). Powder-based 3D printing for bone tissue engineering. Biotechnol. Adv. 34 (5), 740–753. doi: 10.1016/j.biotechadv.2016.03.009
PubMed Abstract | CrossRef Full Text | Google Scholar
Carve, M., Wlodkowic, D. (2018). 3D-Printed chips: compatibility of additive manufacturing photopolymeric substrata with biological applications. Micromachines (Basel) 9 (2), E91. doi: 10.3390/mi9020091
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, H. J., Miller, P., Shuler, M. L. (2018). A pumpless body-on-a-chip model using a primary culture of human intestinal cells and a 3D culture of liver cells. Lab. Chip 18 (14), 2036–2046. doi: 10.1039/c8lc00111a
doi: 10.1039/c8lc00111a
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, Y. R., Zhou, Z. X., Zhang, J. Y., Yuan, F. Z., Xu, B. B., Guan, J., et al. (2019). Low-molecular-weight heparin-functionalized chitosan-chondroitin sulfate hydrogels for controlled release of TGF-beta3 and in vitro neocartilage formation. Front. Chem. 7, 745. doi: 10.3389/fchem.2019.00745
PubMed Abstract | CrossRef Full Text | Google Scholar
Costa, E. C., Moreira, A. F., de Melo-Diogo, D., Gaspar, V. M., Carvalho, M. P., Correia, I. J. (2016). 3D tumor spheroids: an overview on the tools and techniques used for their analysis. Biotechnol. Adv. 34 (8), 1427–1441. doi: 10.1016/j.biotechadv.2016.11.002
PubMed Abstract | CrossRef Full Text | Google Scholar
Credi, C., Fiorese, A., Tironi, M., Bernasconi, R., Magagnin, L., Levi, M., et al. (2016). 3D Printing of cantilever-type microstructures by stereolithography of ferromagnetic photopolymers. ACS Appl. Mater Interfaces 8 (39), 26332–26342. doi: 10.1021/acsami.6b08880
Mater Interfaces 8 (39), 26332–26342. doi: 10.1021/acsami.6b08880
PubMed Abstract | CrossRef Full Text | Google Scholar
Credi, C., Griffini, G., Levi, M., Turri, S. (2018). Biotinylated photopolymers for 3d-printed unibody lab-on-a-chip optical platforms. Small 14 (1), 1702831. doi: 10.1002/smll.201702831
CrossRef Full Text | Google Scholar
Dittmann, W., Bill, J., Wittenberg, G., Reuther, J., Roosen, K. (1994). [Stereolithography as a new method of reconstructive surgical planning in complex osseous defects of the cranial base. Tech. note]. Zentralbl Neurochir 55 (4), 209–211.
Google Scholar
Eltorai, A. E., Nguyen, E., Daniels, A. H. (2015). Three-dimensional printing in orthopedic surgery. Orthopedics 38 (11), 684–687. doi: 10.3928/01477447-20151016-05
PubMed Abstract | CrossRef Full Text | Google Scholar
Fedore, C. W., Tse, L. Y. L., Nam, H. K., Barton, K. L., Hatch, N. E. (2017). Analysis of polycaprolactone scaffolds fabricated via precision extrusion deposition for control of craniofacial tissue mineralization. Orthod. Craniofac. Res. 20 Suppl 1, 12–17. doi: 10.1111/ocr.12159
Orthod. Craniofac. Res. 20 Suppl 1, 12–17. doi: 10.1111/ocr.12159
PubMed Abstract | CrossRef Full Text | Google Scholar
Fina, F., Goyanes, A., Gaisford, S., Basit, A. W. (2017). Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 529 (1-2), 285–293. doi: 10.1016/j.ijpharm.2017.06.082
PubMed Abstract | CrossRef Full Text | Google Scholar
Ganguli, A., Pagan-Diaz, G. J., Grant, L., Cvetkovic, C., Bramlet, M., Vozenilek, J., et al. (2018). 3D printing for preoperative planning and surgical training: a review. BioMed. Microdevices 20 (3), 65. doi: 10.1007/s10544-018-0301-9
PubMed Abstract | CrossRef Full Text | Google Scholar
Giannopoulos, A. A., Mitsouras, D., Yoo, S. J., Liu, P. P., Chatzizisis, Y. S., Rybicki, F. J. (2016). Applications of 3D printing in cardiovascular diseases. Nat. Rev. Cardiol. 13 (12), 701–718. doi: 10.1038/nrcardio.2016.170
PubMed Abstract | CrossRef Full Text | Google Scholar
Graham, A. D., Olof, S. N., Burke, M. J., Armstrong, J. P. K., Mikhailova, E. A., Nicholson, J. G., et al. (2017). High-resolution patterned cellular constructs by droplet-based 3D printing. Sci. Rep. 7 (1), 7004. doi: 10.1038/s41598-017-06358-x
D., Olof, S. N., Burke, M. J., Armstrong, J. P. K., Mikhailova, E. A., Nicholson, J. G., et al. (2017). High-resolution patterned cellular constructs by droplet-based 3D printing. Sci. Rep. 7 (1), 7004. doi: 10.1038/s41598-017-06358-x
PubMed Abstract | CrossRef Full Text | Google Scholar
Han, Y., Yang, S., Huang, W., Wang, Z., Li, H. (2018). A hem-o-lok-induced tracheoesophageal fistula cured by temporary airway stenting modified with three-dimensional printing. Ann. Thorac. Surg. 106 (4), e219–e221. doi: 10.1016/j.athoracsur.2018.04.037
PubMed Abstract | CrossRef Full Text | Google Scholar
Heidari Keshel, S., Rostampour, M., Khosropour, G., Bandbon, B. A., Baradaran-Rafii, A., Biazar, E. (2016). Derivation of epithelial-like cells from eyelid fat-derived stem cells in thermosensitive hydrogel. J. Biomater. Sci. Polym. Ed. 27 (4), 339–350. doi: 10.1080/09205063.2015.1130406
PubMed Abstract | CrossRef Full Text | Google Scholar
Hong, D. , Chou, D. T., Velikokhatnyi, O. I., Roy, A., Lee, B., Swink, I., et al. (2016). Binder-jetting 3D printing and alloy development of new biodegradable Fe-Mn-Ca/Mg alloys. Acta Biomater. 45, 375–386. doi: 10.1016/j.actbio.2016.08.032
, Chou, D. T., Velikokhatnyi, O. I., Roy, A., Lee, B., Swink, I., et al. (2016). Binder-jetting 3D printing and alloy development of new biodegradable Fe-Mn-Ca/Mg alloys. Acta Biomater. 45, 375–386. doi: 10.1016/j.actbio.2016.08.032
PubMed Abstract | CrossRef Full Text | Google Scholar
Hung, K. C., Tseng, C. S., Dai, L. G., Hsu, S. H. (2016). Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. Biomaterials 83, 156–168. doi: 10.1016/j.biomaterials.2016.01.019
PubMed Abstract | CrossRef Full Text | Google Scholar
Ishutov, S., Hasiuk, F. J., Jobe, D., Agar, S. (2018). Using resin-based 3D printing to build geometrically accurate proxies of porous sedimentary rocks. Ground Water 56 (3), 482–490. doi: 10.1111/gwat.12601
PubMed Abstract | CrossRef Full Text | Google Scholar
Jacobs, C. A., Lin, A. Y. (2017). A new classification of three-dimensional printing technologies: systematic review of three-dimensional printing for patient-specific craniomaxillofacial surgery. Plast. Reconstr. Surg. 139 (5), 1211–1220. doi: 10.1097/PRS.0000000000003232
Plast. Reconstr. Surg. 139 (5), 1211–1220. doi: 10.1097/PRS.0000000000003232
PubMed Abstract | CrossRef Full Text | Google Scholar
Jamroz, W., Szafraniec, J., Kurek, M., Jachowicz, R. (2018). 3D Printing in pharmaceutical and medical applications - recent achievements and challenges. Pharm. Res. 35 (9), 176. doi: 10.1007/s11095-018-2454-x
PubMed Abstract | CrossRef Full Text | Google Scholar
Kim, B. S., Kwon, Y. W., Kong, J. S., Park, G. T., Gao, G., Han, W., et al. (2018). 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: a step towards advanced skin tissue engineering. Biomaterials 168, 38–53. doi: 10.1016/j.biomaterials.2018.03.040
PubMed Abstract | CrossRef Full Text | Google Scholar
Kim, D. H., Lee, J., Bae, J., Park, S., Choi, J., Lee, J. H., et al. (2018). Mechanical analysis of ceramic/polymer composite with mesh-type lightweight design using binder-Jet 3D printing. Materials (Basel) 11 (10), E1941. doi: 10.3390/ma11101941
Materials (Basel) 11 (10), E1941. doi: 10.3390/ma11101941
PubMed Abstract | CrossRef Full Text | Google Scholar
Konta, A. A., Garcia-Pina, M., Serrano, D. R. (2017). Personalised 3D printed medicines: which techniques and polymers are more successful? Bioengineering. (Basel) 4 (4), E79. doi: 10.3390/bioengineering4040079
PubMed Abstract | CrossRef Full Text | Google Scholar
Laronda, M. M., Rutz, A. L., Xiao, S., Whelan, K. A., Duncan, F. E., Roth, E. W., et al. (2017). A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat. Commun. 8, 15261. doi: 10.1038/ncomms15261
PubMed Abstract | CrossRef Full Text | Google Scholar
Lau, I. W. W., Liu, D., Xu, L., Fan, Z., Sun, Z. (2018). Clinical value of patient-specific three-dimensional printing of congenital heart disease: quantitative and qualitative assessments. PLoS One 13 (3), e0194333. doi: 10.1371/journal.pone. 0194333
0194333
PubMed Abstract | CrossRef Full Text | Google Scholar
Lee, H. J., Son, M. J., Ahn, J., Oh, S. J., Lee, M., Kim, A., et al. (2017). Elasticity-based development of functionally enhanced multicellular 3D liver encapsulated in hybrid hydrogel. Acta Biomater. 64, 67–79. doi: 10.1016/j.actbio.2017.09.041
PubMed Abstract | CrossRef Full Text | Google Scholar
Legocki, A. T., Duffy-Peter, A., Scott, A. R. (2017). Benefits and limitations of entry-level 3-dimensional printing of maxillofacial skeletal models. JAMA Otolaryngol Head Neck Surg. 143 (4), 389–394. doi: 10.1001/jamaoto.2016.3673
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, J., Zhou, Y., Chen, W., Yuan, Z., You, B., Liu, Y., et al. (2018). A novel 3D in vitro tumor model based on silk fibroin/chitosan scaffolds to mimic the tumor microenvironment. ACS Appl. Mater. Interfaces 10 (43), 36641–36651. doi: 10.1021/acsami.8b10679
PubMed Abstract | CrossRef Full Text | Google Scholar
Liaw, C. Y., Guvendiren, M. (2017). Current and emerging applications of 3D printing in medicine. Biofabrication 9 (2), 024102. doi: 10.1088/1758-5090/aa7279
Y., Guvendiren, M. (2017). Current and emerging applications of 3D printing in medicine. Biofabrication 9 (2), 024102. doi: 10.1088/1758-5090/aa7279
PubMed Abstract | CrossRef Full Text | Google Scholar
Lind, J. U., Busbee, T. A., Valentine, A. D., Pasqualini, F. S., Yuan, H., Yadid, M., et al. (2017). Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater 16 (3), 303–308. doi: 10.1038/nmat4782
PubMed Abstract | CrossRef Full Text | Google Scholar
Mahajan, A., Frisbie, C. D., Francis, L. F. (2013). Optimization of aerosol jet printing for high-resolution, high-aspect ratio silver lines. ACS Appl. Mater. Interfaces 5 (11), 4856–4864. doi: 10.1021/am400606y
PubMed Abstract | CrossRef Full Text | Google Scholar
Mahmood, F., Owais, K., Taylor, C., Montealegre-Gallegos, M., Manning, W., Matyal, R., et al. (2015). Three-dimensional printing of mitral valve using echocardiographic data. JACC Cardiovasc. Imaging 8 (2), 227–229. doi: 10.1016/j.jcmg.2014.06.020
JACC Cardiovasc. Imaging 8 (2), 227–229. doi: 10.1016/j.jcmg.2014.06.020
PubMed Abstract | CrossRef Full Text | Google Scholar
MarketsandMarkets (2019). 3D printing market by offering, process, application, vertical, technology, and geography - global forecast to 2024. Retrieved from https://www.marketsandmarkets.com/Market-Reports/3d-printing-market-1276.html.
Google Scholar
Markstedt, K., Escalante, A., Toriz, G., Gatenholm, P. (2017). Biomimetic inks based on cellulose nanofibrils and cross-linkable xylans for 3d printing. ACS Appl. Mater. Interfaces 9 (46), 40878–40886. doi: 10.1021/acsami.7b13400
PubMed Abstract | CrossRef Full Text | Google Scholar
Mitsouras, D., Liacouras, P., Imanzadeh, A., Giannopoulos, A. A., Cai, T., Kumamaru, K. K., et al. (2015). Medical 3D printing for the radiologist. Radiographics 35 (7), 1965–1988. doi: 10.1148/rg.2015140320
PubMed Abstract | CrossRef Full Text | Google Scholar
Mondschein, R. J., Kanitkar, A., Williams, C. B., Verbridge, S. S., Long, T. E. (2017). Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials 140, 170–188. doi: 10.1016/j.biomaterials.2017.06.005
J., Kanitkar, A., Williams, C. B., Verbridge, S. S., Long, T. E. (2017). Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials 140, 170–188. doi: 10.1016/j.biomaterials.2017.06.005
PubMed Abstract | CrossRef Full Text | Google Scholar
Mukherjee, P., Cheng, K., Flanagan, S., Greenberg, S. (2017). Utility of 3D printed temporal bones in pre-surgical planning for complex BoneBridge cases. Eur. Arch. Otorhinolaryngol. 274 (8), 3021–3028. doi: 10.1007/s00405-017-4618-4
PubMed Abstract | CrossRef Full Text | Google Scholar
Murphy, S. V., Atala, A. (2014). 3D bioprinting of tissues and organs. Nat. Biotechnol. 32 (8), 773–785. doi: 10.1038/nbt.2958
PubMed Abstract | CrossRef Full Text | Google Scholar
Nie, W., Gu, F., Wang, Z., Wu, R., Yue, Y., Shao, A. (2019). Preliminary application of three-dimension printing technology in surgical management of bicondylar tibial plateau fractures. Injury 50 (2), 476–483. doi: 10.1016/j.injury.2018.12.019
Injury 50 (2), 476–483. doi: 10.1016/j.injury.2018.12.019
PubMed Abstract | CrossRef Full Text | Google Scholar
Ning, L., Chen, X. (2017). A brief review of extrusion-based tissue scaffold bio-printing. Biotechnol. J. 12 (8), 1600671. doi: 10.1002/biot.201600671
CrossRef Full Text | Google Scholar
Norman, J., Madurawe, R. D., Moore, C. M., Khan, M. A., Khairuzzaman, A. (2017). A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Delivery Rev. 108, 39–50. doi: 10.1016/j.addr.2016.03.001
CrossRef Full Text | Google Scholar
Olivieri, L. J., Krieger, A., Loke, Y. H., Nath, D. S., Kim, P. C., Sable, C. A. (2015). Three-dimensional printing of intracardiac defects from three-dimensional echocardiographic images: feasibility and relative accuracy. J. Am. Soc. Echocardiogr. 28 (4), 392–397. doi: 10.1016/j.echo.2014.12.016
PubMed Abstract | CrossRef Full Text | Google Scholar
Orsi, G., De Maria, C. , Montemurro, F., Chauhan, V. M., Aylott, J. W., Vozzi, G. (2015). Combining inkjet printing and sol-gel chemistry for making pH-sensitive surfaces. Curr. Top. Med. Chem. 15 (3), 271–278. doi: 10.2174/1568026614666141229114738
, Montemurro, F., Chauhan, V. M., Aylott, J. W., Vozzi, G. (2015). Combining inkjet printing and sol-gel chemistry for making pH-sensitive surfaces. Curr. Top. Med. Chem. 15 (3), 271–278. doi: 10.2174/1568026614666141229114738
PubMed Abstract | CrossRef Full Text | Google Scholar
Osman, R. B., Alharbi, N., Wismeijer, D. (2017). Build angle: does it influence the accuracy of 3d-printed dental restorations using digital light-processing technology? Int. J. Prosthodont. 30 (2), 182–188. doi: 10.11607/ijp.5117
PubMed Abstract | CrossRef Full Text | Google Scholar
Ozbolat, I. T., Hospodiuk, M. (2016). Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 76, 321–343. doi: 10.1016/j.biomaterials.2015.10.076
PubMed Abstract | CrossRef Full Text | Google Scholar
Paxton, N., Smolan, W., Bock, T., Melchels, F., Groll, J., Jungst, T. (2017). Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 9 (4), 044107. doi: 10.1088/1758-5090/aa8dd8
Biofabrication 9 (4), 044107. doi: 10.1088/1758-5090/aa8dd8
PubMed Abstract | CrossRef Full Text | Google Scholar
Petta, D., Armiento, A. R., Grijpma, D., Alini, M., Eglin, D., D'Este, M. (2018). 3D bioprinting of a hyaluronan bioink through enzymatic-and visible light-crosslinking. Biofabrication 10 (4), 044104. doi: 10.1088/1758-5090/aadf58
PubMed Abstract | CrossRef Full Text | Google Scholar
Placone, J. K., Engler, A. J. (2018). Recent advances in extrusion-based 3D printing for biomedical applications. Adv. Healthc Mater 7 (8), e1701161. doi: 10.1002/adhm.201701161
PubMed Abstract | CrossRef Full Text | Google Scholar
Rahman, Z., Barakh Ali, S. F., Ozkan, T., Charoo, N. A., Reddy, I. K., Khan, M. A. (2018). Additive manufacturing with 3D printing: progress from bench to bedside. AAPS J. 20 (6), 101. doi: 10.1208/s12248-018-0225-6
PubMed Abstract | CrossRef Full Text | Google Scholar
Rashidi, H., Luu, N. T., Alwahsh, S. M., Ginai, M., Alhaque, S., Dong, H., et al. (2018). 3D human liver tissue from pluripotent stem cells displays stable phenotype in vitro and supports compromised liver function in vivo. Arch. Toxicol. 92 (10), 3117–3129. doi: 10.1007/s00204-018-2280-2
T., Alwahsh, S. M., Ginai, M., Alhaque, S., Dong, H., et al. (2018). 3D human liver tissue from pluripotent stem cells displays stable phenotype in vitro and supports compromised liver function in vivo. Arch. Toxicol. 92 (10), 3117–3129. doi: 10.1007/s00204-018-2280-2
PubMed Abstract | CrossRef Full Text | Google Scholar
Revilla-Leon, M., Ozcan, M. (2019). Additive manufacturing technologies used for processing polymers: current status and potential application in prosthetic dentistry. J. Prosthodont. 28 (2), 146–158. doi: 10.1111/jopr.12801
PubMed Abstract | CrossRef Full Text | Google Scholar
Serex, L., Bertsch, A., Renaud, P. (2018). Microfluidics: a new layer of control for extrusion-based 3d printing. Micromachines (Basel) 9 (2), E86. doi: 10.3390/mi9020086
PubMed Abstract | CrossRef Full Text | Google Scholar
Smith, M. L., Jones, J. F. X. (2018). Dual-extrusion 3D printing of anatomical models for education. Anat. Sci. Educ. 11 (1), 65–72. doi: 10.1002/ase.1730
Anat. Sci. Educ. 11 (1), 65–72. doi: 10.1002/ase.1730
PubMed Abstract | CrossRef Full Text | Google Scholar
Son, K. H., Kim, K. W., Ahn, C. B., Choi, C. H., Park, K. Y., Park, C. H., et al. (2015). Surgical Planning by 3D Printing for Primary Cardiac Schwannoma Resection. Yonsei Med. J. 56 (6), 1735–1737. doi: 10.3349/ymj.2015.56.6.1735
PubMed Abstract | CrossRef Full Text | Google Scholar
Stefaniak, A. B., Bowers, L. N., Knepp, A. K., Luxton, T. P., Peloquin, D. M., Baumann, E. J., et al. (2019). Particle and vapor emissions from vat polymerization desktop-scale 3-dimensional printers. J. Occup. Environ. Hyg. 16 (8), 519–531. doi: 10.1080/15459624.2019.1612068
PubMed Abstract | CrossRef Full Text | Google Scholar
Su, W., Xiao, Y., He, S., Huang, P., Deng, X. (2018). Three-dimensional printing models in congenital heart disease education for medical students: a controlled comparative study. BMC Med. Educ. 18 (1), 178. doi: 10. 1186/s12909-018-1293-0
1186/s12909-018-1293-0
PubMed Abstract | CrossRef Full Text | Google Scholar
Tahayeri, A., Morgan, M., Fugolin, A. P., Bompolaki, D., Athirasala, A., Pfeifer, C. S., et al. (2018). 3D printed versus conventionally cured provisional crown and bridge dental materials. Dent. Mater. 34 (2), 192–200. doi: 10.1016/j.dental.2017.10.003
PubMed Abstract | CrossRef Full Text | Google Scholar
Taylor, S. L., Ibeh, A. J., Jakus, A. E., Shah, R. N., Dunand, D. C. (2018). NiTi-Nb micro-trusses fabricated via extrusion-based 3D-printing of powders and transient-liquid-phase sintering. Acta Biomater. 76, 359–370. doi: 10.1016/j.actbio.2018.06.015
PubMed Abstract | CrossRef Full Text | Google Scholar
Trenfield, S. J., Awad, A., Goyanes, A., Gaisford, S., Basit, A. W. (2018). 3D Printing pharmaceuticals: drug development to frontline care. Trends Pharmacol. Sci. 39 (5), 440–451. doi: 10.1016/j.tips.2018.02.006
PubMed Abstract | CrossRef Full Text | Google Scholar
VanKoevering, K. K., Morrison, R. J., Prabhu, S. P., Torres, M. F., Mychaliska, G. B., Treadwell, M. C., et al. (2015). Antenatal three-dimensional printing of aberrant facial anatomy. Pediatrics 136 (5), e1382–e1385. doi: 10.1542/peds.2015-1062
K., Morrison, R. J., Prabhu, S. P., Torres, M. F., Mychaliska, G. B., Treadwell, M. C., et al. (2015). Antenatal three-dimensional printing of aberrant facial anatomy. Pediatrics 136 (5), e1382–e1385. doi: 10.1542/peds.2015-1062
PubMed Abstract | CrossRef Full Text | Google Scholar
Vukicevic, M., Mosadegh, B., Min, J. K., Little, S. H. (2017). Cardiac 3D Printing and its Future Directions. JACC Cardiovasc. Imaging 10 (2), 171–184. doi: 10.1016/j.jcmg.2016.12.001
PubMed Abstract | CrossRef Full Text | Google Scholar
Wang, X., Gao, P., Yang, Y., Guo, H., Wu, D. (2018a). Dynamic and programmable morphology and size evolution via a living hierarchical self-assembly strategy. Nat. Commun. 9 (1), 2772. doi: 10.1038/s41467-018-05142-3
PubMed Abstract | CrossRef Full Text | Google Scholar
Wang, X., Yang, Y., Fan, L., Yang, F., Wu, D. (2018b). POSS-embedded supramolecular hyperbranched polymers constructed from a 1→7 branching monomer with controllable morphology transitions. China Chem. 61, 3, 311–318. doi: 10.1007/s11426-017-9168-3
China Chem. 61, 3, 311–318. doi: 10.1007/s11426-017-9168-3
CrossRef Full Text | Google Scholar
Wang, S. J., Jiang, D., Zhang, Z. Z., Chen, Y. R., Yang, Z. D., Zhang, J. Y., et al. (2019). Biomimetic nanosilica-collagen scaffolds for in situ bone regeneration: toward a cell-free, one-step surgery. Adv. Mater. 31 (49), e1904341. doi: 10.1002/adma.201904341
PubMed Abstract | CrossRef Full Text | Google Scholar
Wei, R., Guo, W., Ji, T., Zhang, Y., Liang, H. (2017). One-step reconstruction with a 3D-printed, custom-made prosthesis after total en bloc sacrectomy: a technical note. Eur. Spine J. 26 (7), 1902–1909. doi: 10.1007/s00586-016-4871-z
PubMed Abstract | CrossRef Full Text | Google Scholar
Weinhart, M., Hocke, A., Hippenstiel, S., Kurreck, J., Hedtrich, S. (2019). 3D organ models-Revolution in pharmacological research? Pharmacol. Res. 139, 446–451. doi: 10.1016/j.phrs.2018.11.002
PubMed Abstract | CrossRef Full Text | Google Scholar
Wills, J. W., Hondow, N., Thomas, A. D., Chapman, K. E., Fish, D., Maffeis, T. G., et al. (2016). Genetic toxicity assessment of engineered nanoparticles using a 3D in vitro skin model (EpiDerm). Part Fibre Toxicol. 13 (1), 50. doi: 10.1186/s12989-016-0161-5
W., Hondow, N., Thomas, A. D., Chapman, K. E., Fish, D., Maffeis, T. G., et al. (2016). Genetic toxicity assessment of engineered nanoparticles using a 3D in vitro skin model (EpiDerm). Part Fibre Toxicol. 13 (1), 50. doi: 10.1186/s12989-016-0161-5
PubMed Abstract | CrossRef Full Text | Google Scholar
Wollhofen, R., Axmann, M., Freudenthaler, P., Gabriel, C., Rohrl, C., Stangl, H., et al. (2018). Multiphoton-Polymerized 3D Protein Assay. ACS Appl. Mater. Interfaces 10 (2), 1474–1479. doi: 10.1021/acsami.7b13183
PubMed Abstract | CrossRef Full Text | Google Scholar
Wong, J. Y. (2016). 3D printing applications for space missions. Aerosp Med. Hum. Perform. 87 (6), 580–582. doi: 10.3357/AMHP.4633.2016
PubMed Abstract | CrossRef Full Text | Google Scholar
Wysocki, B., Idaszek, J., Zdunek, J., Rozniatowski, K., Pisarek, M., Yamamoto, A., et al. (2018). The influence of selective laser melting (SLM) process parameters on in-vitro cell response. Int. J. Mol. Sci., 19 (6), E1619. doi: 10.3390/ijms19061619
Int. J. Mol. Sci., 19 (6), E1619. doi: 10.3390/ijms19061619
PubMed Abstract | CrossRef Full Text | Google Scholar
Xu, N., Wei, F., Liu, X., Jiang, L., Cai, H., Li, Z., et al. (2016). Reconstruction of the upper cervical spine using a personalized 3d-printed vertebral body in an adolescent with ewing sarcoma. Spine (Phila Pa 1976) 41 (1), E50–E54. doi: 10.1097/BRS.0000000000001179
PubMed Abstract | CrossRef Full Text | Google Scholar
Yang, Y., Wang, X., Yang, F., Wang, L., Wu, D. (2018). Highly elastic and ultratough hybrid ionic-covalent hydrogels with tunable structures and mechanics. Adv. Mater. 30 (18), e1707071. doi: 10.1002/adma.201707071
PubMed Abstract | CrossRef Full Text | Google Scholar
Yuan, B., Zhou, S. Y., Chen, X. S. (2017). Rapid prototyping technology and its application in bone tissue engineering. J. Zhejiang Univ. Sci. B 18 (4), 303–315. doi: 10.1631/jzus.B1600118
PubMed Abstract | CrossRef Full Text | Google Scholar
Zanchetta, E. , Cattaldo, M., Franchin, G., Schwentenwein, M., Homa, J., Brusatin, G., et al. (2016). Stereolithography of SiOC ceramic microcomponents. Adv. Mater. 28 (2), 370–376. doi: 10.1002/adma.201503470
, Cattaldo, M., Franchin, G., Schwentenwein, M., Homa, J., Brusatin, G., et al. (2016). Stereolithography of SiOC ceramic microcomponents. Adv. Mater. 28 (2), 370–376. doi: 10.1002/adma.201503470
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhao, Y., Yao, R., Ouyang, L., Ding, H., Zhang, T., Zhang, K., et al. (2014). Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 6 (3), 035001. doi: 10.1088/1758-5082/6/3/035001
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhu, C., Xia, Y., Zai, Y., Dai, Y., Liu, X., Bian, J., et al. (2019). Adsorption and desorption behaviors of HPEI and thermoresponsive HPEI based gels on anionic and cationic dyes. Chem. Eng. J. 369, 863–873. doi: 10.1016/j.cej.2019.03.169
CrossRef Full Text | Google Scholar
Zopf, D. A., Flanagan, C. L., Wheeler, M., Hollister, S. J., Green, G. E. (2014). Treatment of severe porcine tracheomalacia with a 3-dimensionally printed, bioresorbable, external airway splint. JAMA Otolaryngol. Head Neck Surg. 140 (1), 66–71. doi: 10.1001/jamaoto.2013.5644
JAMA Otolaryngol. Head Neck Surg. 140 (1), 66–71. doi: 10.1001/jamaoto.2013.5644
PubMed Abstract | CrossRef Full Text | Google Scholar
3D printing to solve pre-volume production problems
Google Advanced Technology Projects Lab (ATAP) is a one-stop shop for hardware projects. The lab has a forward-thinking approach to product and manufacturing, and employs problem-solving approaches that any company can adopt today, from iterative thinking at every stage of development to technology tools that allow flexibility and creativity in problem solving.
In one case, this approach led to a technological innovation that eliminated a complex supply chain in the model validation phase in preparation for mass production of a handheld device made using overmolding technology. Using Formlabs High Temp Resin, a 3D printing material with high thermal resistance, they bridged the gap between prototyping and production, cutting key component manufacturing time by 85% and saving over $100,000 in the process.
Watch the video to see how Google ATAP used additive manufacturing to bridge the gap between prototyping and production, using Formlabs 3D printers and High Temp Resin to solve the problem of preparing for mass production of a complex handheld device made with technology reformulation.
“We are trying to understand not only what products will look like in the future, but also what production will be like. Additive manufacturing really plays an important role in this and is directly related to many of the projects we work on,” says Brian Allen, Google ATAP design technologist who specializes in 3D printing and advanced manufacturing technologies.
“I’m excited when we can discover something new that’s happening in 3D printing — a new material, a new process — and then apply it to the designs we build in new ways, doing something more efficiently, faster, better. or more aesthetic.
Our employees didn't know what to do. They were preparing to mass-produce a portable device made using overmolding technology and needed faster results. The process was new, the supply chain was complex, and they had to overcome these complexities and get the product ready for delivery.
The process was new, the supply chain was complex, and they had to overcome these complexities and get the product ready for delivery.
“We had electronic components that were re-moulded and then re-moulded, and the result was this flexible, waterproof item that we could use in a portable device,” says David Beardsley, Experimental Workshop Manager at Google ATAP Lab. .
Remolding is a common manufacturing process. If we talk about a part without electronic components, made by the overmolding method, the plant can produce thousands of prototypes at a very low cost. But ATAP laboratory specialists re-formed an already re-formed electronic component - a printed circuit board module with complex electronics that was supplied from another factory. Therefore, prototypes were expensive, and their production depended on how quickly the first plant could produce and ship circuit board modules.
Re-molding is an injection molding process that usually requires initial tooling at the factory for new parts, which are called prototypes. As casting parameters are calibrated, these prototypes may be underfilled, overfilled, or have cosmetic imperfections. When troubleshooting, process engineers ensure cutoffs, pressures, and all forming parameters are correct.
As casting parameters are calibrated, these prototypes may be underfilled, overfilled, or have cosmetic imperfections. When troubleshooting, process engineers ensure cutoffs, pressures, and all forming parameters are correct.
Manufacture of a portable device starts by adding components to the printed circuit board. This circuit board is encapsulated in a low pressure molding system that turns it into a plastic block. The printed circuit board and flexible cable form an electronic assembly that undergoes a re-moulding process with thermoplastic polyurethane (TPU) and silicone rubber. The PCB module then undergoes a final reshaping.
During pre-production, the employees realized that the cost of prototypes would be much higher than for a conventional injection molded part. But they didn't expect supply chain problems: it took three weeks to get the remolded electronic assemblies needed to carry out these tests. Beardsley needed to shorten this time to ramp up production and deliver finished products.
“It can take hundreds or thousands of samples to perfect a product. But the problem is that if you do this with working electronics with real boards that were stuffed with electronic components, sent for reformulation, and then returned back, you get a whole supply chain, ”explains Beardsley.
“I ran out of ideas and just watched dollars and weeks go to waste while we tried to figure out how to get the tool right. How do we prove that it will work before we start putting working electronic components in there?”
We had to find a process and material that would be suitable for the PCB module. The replacement had to be both dimensionally accurate and fully shaped to the real part so that the characteristics of the filler could be determined, and strong enough to be able to cut off without breaking or deflecting it, resulting in burrs.
“We knew we had to use a material that could withstand hundreds of Newtons of pressure at over 250 °C,” says Beardsley. “We were looking for a material with high stiffness and high temperature resistance. ”
”
Beardsley turned to Allen for help and they came up with a plan.
“We knew we had to use a material that could withstand pressures of hundreds of newtons at over 250 °C. We were looking for a material with high rigidity and high temperature resistance”
David Beardsley
The parameters left no room for maneuver. Allen decided to try printing replacements (replacement parts) from High Temp Resin on a Form 2 stereolithographic (SLA) 3D printer. He knew he would be testing the limits of this material: the finished parts had to be injected at 270°C and over 1,800 bar—above the cut-off temperature specified for High Temp Resin.
Explore new formulations and other uses for this heat resistant 3D printing material or request a free sample part.
Request a free sample
“In order to get the small part size and cutoffs we needed, we needed this resolution. It was the combination of resolution and high temperature that allowed us to use the Form 2 printer for this,” says Allen. “We have many other manufacturing technologies to choose from, but being able to make these parts is very important to our lab.”
“We have many other manufacturing technologies to choose from, but being able to make these parts is very important to our lab.”
“We have many other manufacturing technologies to choose from, but being able to make these parts is very important to our lab.”
Brian Allen
The staff quickly got to work, printing a certain number of models at night.
“We didn't have time to redo the CAD. I opened it, exported the STL file, and loaded it into PreForm. As soon as we received the first batch for confirmation, we just launched it. In the first cycle we made 200 parts, and then another 100,” says Allen.
PreForm is Formlabs free model preparation software that can be used to build batches of models on the platform. As they picked up the pace, Allen printed 250 replacement parts in batches of 10, each taking about four hours, allowing employees to produce hundreds of parts over the weekend.
Allen installed the supports on the High Temp Resin models in such a way that the traces of them remained only on the models, and not on the molding surfaces. This ensures that the parts will not require additional sanding or finishing beyond the standard wash and final cure before use.
This ensures that the parts will not require additional sanding or finishing beyond the standard wash and final cure before use.
3D printed models are ideal for replacing electronic components. This process reduced the production time for replacement parts from three weeks to three days, and reduced the cost per replacement part from $100 to 80 cents.
“This allowed us to intercept the process in the subsequent stages of the project and eliminate a number of preparatory steps. Due to this, we simply abandoned three or four preparatory stages. It saved a lot of time,” says Beardsley.
David Beardsley shows how his team was able to "hijack the process," as he puts it, by eliminating four production steps, allowing them to focus on quickly perfecting the model in the final remolding step. 3D-printed replacement parts eliminated a complex supply chain and cut production time from three weeks to three days.
“We were able to cut off a 3D printed part and inject high pressure materials into it without igniting it, which is unique in its own way.
If we didn't have a Form 2 printer, we wouldn't be able to do this."
David Beardsley
Because the printed parts were incredibly cheap, the lab staff were able to provide more than what the factory required so they could continue development until satisfactory results were obtained.
“We were able to cut off a 3D printed part and inject high pressure materials into it without igniting it, which is unique in its own way. If we didn't have a Form 2 printer, we wouldn't be able to do this,” admits Beardsley.
“When we finally moved into full production, we knew it would work,” adds Allen.
Brief: What has Google ATAP achieved? Using a Formlabs Form 2 stereo 3D printer and High Temp Resin, they were able to:
-
3D print High Temp Resin replacement parts that have withstood over-molding and TPU injection at over 250°C and over 1800 bar;
-
to save approximately $100,000 in electronic component manufacturing - and even more when labor costs are included;
-
eliminate a complex supply chain and shorten the test cycle for preparing PCB modules for mass production from 3 weeks to 3 days.

"When we finally moved into full production, we were sure it would work."
Brian Allen
“When we finally moved into full production, we were sure it would work.”
David Beardsley
While the handheld device manufacturing process was unique in this case, the way the lab approached the problem, used technology to solve it, and thought about 3D printing could be a valuable learning experience for companies any size. Check out these three inspiring takeaways from our conversation with Google ATAP Design Technologist Brian Allen and Experimental Workshop Manager David Beardsley.
Approach every stage of development, from prototyping to delivery of the finished product, in terms of iterations.
“There's one really unique thing about our job: we treat the entire manufacturing process as a prototyping process. We run prototyping cycles at every stage of production rather than saying, "OK, here's the prototype stage, here's the supply chain stage, here's the new product launch. " We apply a development process and an iterative approach throughout the product development process, not just at the beginning,” Allen says.
" We apply a development process and an iterative approach throughout the product development process, not just at the beginning,” Allen says.
“When you apply prototyping to every little pre-production step, that's when you get insight and can solve problems before they become big problems,” adds Beardsley.
Don't isolate technology. Treat any piece of equipment as part of a suite of problem-solving tools to help you find the best solution for any task.
"We never try to replace the process completely, we always dig deeper and say, 'OK, what good use can this equipment have? What does this equipment do best, and how can we apply it?' Not focusing on a single process or specific equipment allows us to see the benefits of different materials and processes and apply them accordingly,” says Allen.
“We see 3D printing as another tool along with CNC, injection molding and more traditional manufacturing processes. It helps us think about how to apply these new technologies in production. ”
”
The Form 2 3D Printer is part of the Google ATAP Lab toolkit that engineers and developers use to solve complex problems and implement ambitious hardware projects.
Start by solving one problem.
“There are still so many limitations in part design. For labs that want to start using additive manufacturing: choose a task that is part of a larger problem. Instead of trying to print the entire tool, just try printing a replacement part. Take one task and learn from it,” says Allen.
Find out how a manufacturing engineer at Ashley Furniture brought the idea of 3D printing to life by producing 700 parts in-house.
The Form 2 3D printer and High Temp Resin are among the tools in the Google ATAP lab that engineers and developers use to solve complex problems and realize ambitious hardware projects.
"All the big companies with big workshops are like, 'Oh, you need to buy this expensive equipment.' We have one, and it's really wonderful. But the thing is, we use Form 2 every day,” says Beardsley. “Accuracy, speed, final processing, the ability to quickly get the finished model. It's really impressive."
But the thing is, we use Form 2 every day,” says Beardsley. “Accuracy, speed, final processing, the ability to quickly get the finished model. It's really impressive."
Learn more about Form 2 3D printers and view Formlabs' range of engineering resins to use in your own projects, or request a free 3D print sample to see the quality of High Temp Resin and other resins for yourself.
Explore more 3D printing technologies for prototyping and manufacturing by reading our white paper Making Injection Molds with 3D Models.
Technology: mold making with 3D models
How technology is used in orbit
Stories
Stories
Anna Polyakova
Editor (RB)
Anna Polyakova
3D printing is taking over another industry: aerospace. Not only is this technology changing the way spacecraft are built, but it could also play an important role in the future colonization of other planets.
Not only is this technology changing the way spacecraft are built, but it could also play an important role in the future colonization of other planets.
However, space is a difficult environment to work in, and 3D printing faces a number of challenges in it. In this article, we will look at six main ones.
Anna Polyakova
Article content:
- Imperfect surfaces not suitable for space
- 3D printing in zero gravity becomes more difficult
- Popular FDM printing almost impossible without gravity
- Parts are sometimes sticky
- Not all tools can be 3D printed
- Building "houses" can be a logistical nightmare
Imperfect surfaces not suitable for space
Printing parts and entire spaceships in a 3D printer is a truly exciting prospect. It will reduce waste from production, as well as help create lighter and more fuel-efficient rockets. However, this technology has a serious problem.
It will reduce waste from production, as well as help create lighter and more fuel-efficient rockets. However, this technology has a serious problem.
3D printing produces imperfect surfaces. This becomes apparent when looking at a 3D printed part through a microscope. It may work on Earth, but space requires a different level of accuracy, as errors are almost unacceptable there. A flawed surface can develop cracks and be damaged by countless objects flying through space.
3D printing in zero gravity becomes more difficult
Now we are not saying that 3D printing in space, without gravity at all, is impossible. However, organizing this process is not easy.
The basic design of the 3D printer remains the same, but printing in zero gravity requires special additions. In space, gravity no longer holds the layers of an object together before they cool, so the material itself must be sticky and prevent them from separating from each other.
Popular FDM printing almost impossible without gravity
FDM printing is a well-known standard and can be stumbled upon by typing "3D printing" into Google. If you have a 3D printer, chances are it is an FDM printer. FDM printing is good, but some believe that there are more functional and accurate technologies that use powder or photopolymer.
However, weightlessness makes these processes practically impossible. The lack of gravity makes it difficult to combine parts during printing. However, there are companies that are currently working with NASA to find potential alternatives to FDM printing.
Parts are sometimes sticky
As mentioned above, due to the lack of gravity, 3D printers must hold parts in place and hold layers together during FDM printing. There have been several cases of objects getting stuck on the build plates so hard that both the build plates and the printer were damaged.
Although 3D printers have been successfully printed many times in space, there are occasional issues that indicate that the process is not yet perfect.
Not all tools can be 3D printed
One of the biggest benefits of having a 3D printer on a ship is the ability to travel light into space. All necessary tools and spare parts can be simply printed. But how to figure out what to take with you, and what can a 3D printer handle?
Although researchers do their best to ensure the safety of space flight, people and ships are always exposed to unforeseen factors that are difficult to predict. What to take with you and what to leave behind 3D printing is not an easy choice.
Building "houses" can be a logistical nightmare
When people finally get to planet X, they probably won't want to work in harsh conditions. Perhaps living quarters and laboratories will be created using 3D printers.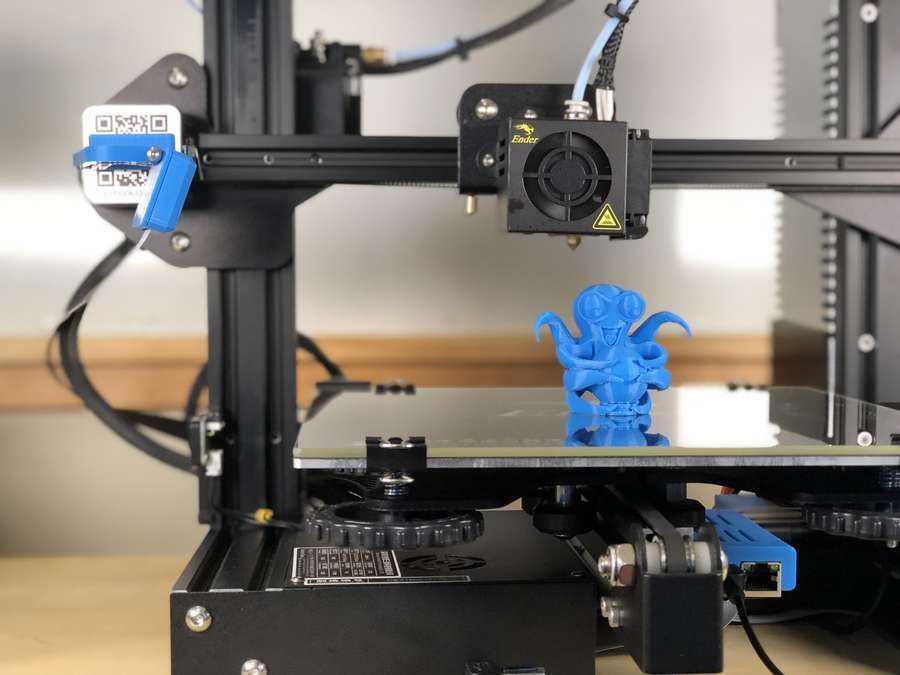 Its use on another planet will be difficult and will require the involvement of robotics and artificial intelligence in the process.
Its use on another planet will be difficult and will require the involvement of robotics and artificial intelligence in the process.
Not to mention that the printer will need to be protected from meteors, temperature changes and any other environmental influences. However, NASA recently held a competition that considered ideas for creating a 3D printing environment for deep space exploration. The results were impressive and solved some of the problems mentioned above.
Source.
Photo: NASA
- 3D printing
- Space
- Technology
Found a typo? Select the text and press Ctrl + Enter
Related materials
- one How to make money on 3D printing: an overview of promising niches
- 2 Organ printing: how 3D bioprinting technologies have advanced and what hinders their development
- 3 3D-printed rocket to launch in 2021
- four “It is allowed to touch the exhibits”: how 3D printing is transforming museums
CAPABILITIES
October 10, 2022
All-Russian competition “Your business.