3D printer living tissue
3D Bioprinting of Living Tissues
Progress in drug testing and regenerative medicine could greatly benefit from laboratory-engineered human tissues built of a variety of cell types with precise 3D architecture. But production of greater than millimeter sized human tissues has been limited by a lack of methods for building tissues with embedded life-sustaining vascular networks.
Play
In this video, the Wyss Institute and Harvard SEAS team uses a customizable 3D bioprinting method to build a thick vascularized tissue structure comprising human stem cells, collective matrix, and blood vessel endothelial cells. Their work sets the stage for advancement of tissue replacement and tissue engineering techniques.Multidisciplinary research at the Wyss Institute has led to the development of a multi-material 3D bioprinting method that generates vascularized tissues composed of living human cells that are nearly ten-fold thicker than previously engineered tissues and that can sustain their architecture and function for upwards of six weeks. The method uses a customizable, printed silicone mold to house and plumb the printed tissue on a chip. Inside this mold, a grid of larger vascular channels containing living endothelial cells in silicone ink is printed, into which a self-supporting ink containing living mesenchymal stem cells (MSCs) is layered in a separate print job. After printing, a liquid composed of fibroblasts and extracellular matrix is used to fill open regions within the construct, adding a connective tissue component that cross-links and further stabilizes the entire structure.
Confocal microscopy image showing a cross-section of a 3D-printed, 1-centimeter-thick vascularized tissue construct showing stem cell differentiation towards development of bone cells, following one month of active perfusion of fluids, nutrients, and cell growth factors.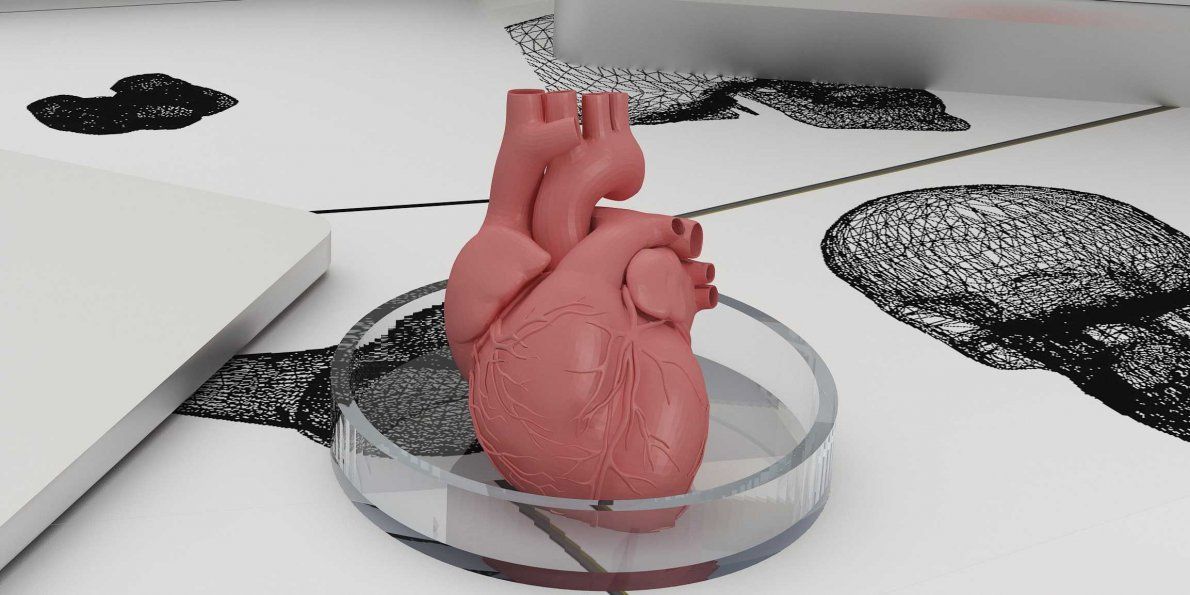 The structure was fabricated using a novel 3D bioprinting strategy invented by Jennifer Lewis and her team at the Wyss Institute and Harvard SEAS. Credit: Lewis Lab, Wyss Institute at Harvard University
The structure was fabricated using a novel 3D bioprinting strategy invented by Jennifer Lewis and her team at the Wyss Institute and Harvard SEAS. Credit: Lewis Lab, Wyss Institute at Harvard UniversityThe resulting soft tissue structure can be immediately perfused with nutrients as well as growth and differentiation factors via a single inlet and outlet on opposite ends of the chip that connect to the vascular channel to ensure survival and maturation of the cells. In a proof-of-principle study, one centimeter thick bioprinted tissue constructs containing human bone marrow MSCs surrounded by connective tissue and supported by an artificial endothelium-lined vasculature, allowed the circulation of bone growth factors and, subsequently, the induction of bone development.
This innovative bioprinting approach can be modified to create various vascularized 3D tissues for regenerative medicine and drug testing endeavors. The Wyss team is also investigating the use of 3D bioprinting to fabricate new versions of the Institute’s organs on chips devices, which makes their manufacturing process more automated and enables development of increasingly complex microphysiological devices.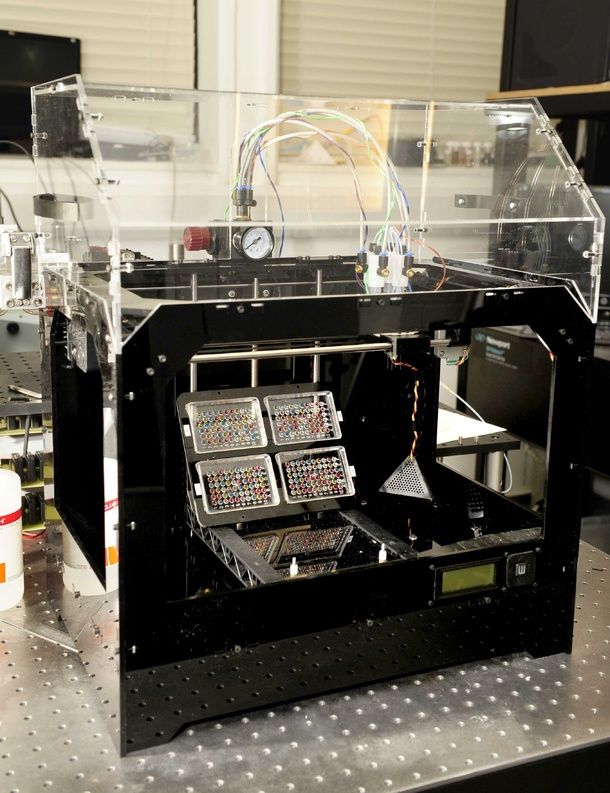 This effort has resulted in the first entirely 3D-printed organ on a chip – a heart on a chip – with integrated soft strain sensors.
This effort has resulted in the first entirely 3D-printed organ on a chip – a heart on a chip – with integrated soft strain sensors.
- 1/7 Cross section of long-term perfusion of HUVEC-lined (red) vascular network supporting HNDFladen (green) matrix.
- 2/7 Top-down view of long-term perfusion of HUVEC-lined (red) vascular network supporting HNDFladen (green) matrix.
- 3/7 Photograph cross section of printed tissue construct housed within a perfusion chamber.
- 4/7 Photograph cross section of printed tissue construct housed within a perfusion chamber.
- 5/7 Photograph of a printed tissue construct housed within a perfusion chamber.
- 6/7 Photograph of vasculature network and cell inks.
- 7/7 Photograph of 3D printed vasculature network (red) within Red is the
- Next
- Prev
6 Advances in 3D Bioprinting of Living Tissue
In recent years, 3D bioprinting has made huge strides toward the goal of printing of organs that can be successfully transplanted into humans. While that’s still far in the future, the method continues to be studied and perfected and advances can lead to new and improved treatments for conditions such as spinal cord injury, Alzheimer’s disease, Parkinson’s disease, brain cancer, and much more.
While that’s still far in the future, the method continues to be studied and perfected and advances can lead to new and improved treatments for conditions such as spinal cord injury, Alzheimer’s disease, Parkinson’s disease, brain cancer, and much more.
The 3D printing of living cells follows standard 3D printing methods, with a few twists. The printer, following a CAD file, lays down layer upon layer of material to build a shape. Instead of metals or plastics, bioprinters use bioinks as their materials. These contain living cells amid viscous materials like alginate or gelatin. The cells are often built upon scaffolding to support and protect the cells.
There have been many recent developments that are pushing the 3D bioprinting field forward. Here are six major advances.
Researchers at Rensselaer Polytechnic Institute and Yale University use a liquid bioink derived from human skin cells to print artificial skin. A blood vessel system then grows naturally within the skin.
A blood vessel system then grows naturally within the skin.
Researchers at Rensselaer Polytechnic Institute and Yale University use a liquid bioink derived from human skin cells to print artificial skin. Image: Rensselaer Polytechnic Institute
With a working vascular system to circulate blood, a patient could assimilate the grafted tissue much more quickly, said Pankaj Karande, a professor of chemical and biological engineering at Rensselaer, who led the research. While bioengineers have had success printing living tissue, few of those few projects have included blood vessels, he said.
“The vasculature is very important because that’s how the host and the graft talk to each other,” Karande said. “Communication between host and graft is critical if the skin substitute is not to be rejected by the body.”
Recommended for You: 3D Printing Overcoming Biocompatibility Challenge
Currently, patients in need of skin grafts have two options: an autologous skin grafts, where the doctors shave off a piece of healthy skin to cover the damaged area; or artificial skin products made from materials that range from bovine collagen to polymer foam.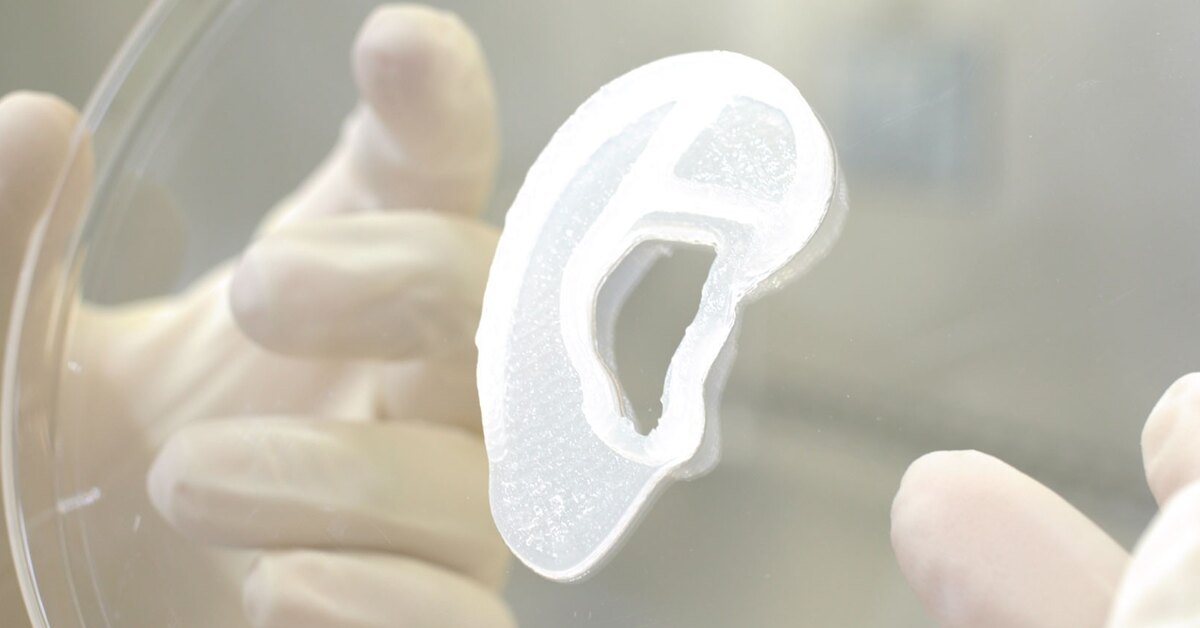 Both have disadvantages. Autologous skin grafts are painful and create a new wound. Artificial skin products have a range of limitations—they are often temporary, don’t cover deep wounds, or don’t resemble human skin, Karande added.
Both have disadvantages. Autologous skin grafts are painful and create a new wound. Artificial skin products have a range of limitations—they are often temporary, don’t cover deep wounds, or don’t resemble human skin, Karande added.
His team’s method is still in the basic research stage, Karande said.
Researchers at Rice University in Houston carve grooves into the plastic threads they use to build scaffolds. They then seed the grooves with cells or other bioactive agents that encourage the growth of new tissue.
The biocompatible implants created by Rice University researchers can degrade over time and leave only natural tissue. Image: Rice University
Unlike the cell-supporting hydrogel scaffolds under development in many labs, this process creates hard implants to be surgically inserted to heal bone, cartilage, or muscle, said Antonios Mikos, a biomedical engineer at Rice, who led the research. The biocompatible implants would degrade over time and leave only natural tissue.
The biocompatible implants would degrade over time and leave only natural tissue.
Usually, 3D-printed scaffolds are seeded with uniform distributions of cells, he said.
“If we wanted different cell populations at different points in the scaffold, we could not do that. Now we can,” he said.
“The major innovation is the capability to spatially load a 3-D printed scaffold with different types of cells populations and with different bioactive molecules,” Mikos said.
This first-of-its-kind method brings the field of tissue engineering one step closer to being able to 3-D print a full-sized, adult human heart, according to the Carnegie Mellon University researchers who created technique.
Scientists from Carnegie Mellon University use 3D bioprinting to build functional parts of the human heart, such as this heart valve. Image: Carnegie Mellon University
Image: Carnegie Mellon University
The technique, known as Freeform Reversible Embedding of Suspended Hydrogels (FRESH), overcomes challenges associated with existing 3-D bioprinting methods. It achieves unprecedented resolution and fidelity using soft and living materials, said Adam Feinberg, a professor of biomedical engineering whose Regenerative Biomaterials and Therapeutics Group performed the work.
Each of the organs in the human body, such as the heart, is built from specialized cells held together by a biological scaffold called the extracellular matrix (ECM). Networked ECM proteins provide the structure and biochemical signals that cells need to carry out their normal function.
Editor’s Pick: VA System Rolls Out 3D Printing
Until now, traditional biofabrication methods couldn’t recreate complex ECM architecture, Feinberg said.
“What we’ve shown is that out of cells and collagen, we can print parts that truly function, like a heart valve or a small beating ventricle,” he said. “By using MRI data of a human heart, we were able to accurately reproduce patient-specific anatomical structures and to 3-D bioprint collagen and human heart cells.”
“By using MRI data of a human heart, we were able to accurately reproduce patient-specific anatomical structures and to 3-D bioprint collagen and human heart cells.”
Collagen is hard to 3-D print because it starts out as a fluid, said Andrew Hudson, a doctoral student in Feinberg’s lab.
“If you try to print this in the air it just forms a puddle on your build platform,” said Hudson. “We’ve developed a technique that prevents it from deforming.”
The FRESH method deposits collagen layer-by-layer within a support bath of gel, giving the collagen a chance to solidify in place before it is removed from the support bath. The supportive gel is melted away by heating the gel from room temperature to body temperature after the print is complete. The researchers can then remove the support gel without damaging the printed structure made of collagen or cells.
A handheld 3D printer that deposits sheets of biomaterial skin to cover large burn wounds is another recent advance. The biomaterial also accelerates the healing process.
The biomaterial also accelerates the healing process.
A handheld 3D printer lays down wound-healing strips of biomaterial to cover burn wounds. Image: University of Toronto
The device, from researchers at the University of Toronto and Sunnybrook Hospital in Toronto, dispenses bio ink over burn wound, strip by strip. The biomaterial is made from bio ink, itself composed of mesenchymal stroma cells (MSCs)—stem cells that differentiate into specialized cell types depending on their environment.
In this case, the MSC material promotes skin regeneration and reduces scarring, says biomedical doctoral candidate Richard Cheng. He heads the project under Axel Guenther, an associate professor of mechanical engineering at the school.
“Previously, we proved that we could deposit cells onto a burn, but there wasn't any proof that there were any wound-healing benefits; now we've demonstrated that,” Guenther said.
When the team unveiled its first prototype of the skin printer in 2018 it was believed to be the first device of its kind to form tissue in situ, depositing and setting tissue in place in two minutes or less.
Patient-specific, 3D-printed bone grafts could create new treatments for patients suffering from arthritis, bone fractures, dental infections, and craniofacial defects, said Akhilesh Gaharwar, associate professor of mechanical engineering at Texas A&M University. His team is leading this research.
Their bioink is an answer to the lack of bioinks that meet the demands of both 3D printing and tissue engineering, he said.
The Texas A&M group’s NICE bioink formulation is used specifically to print 3D bone. Image: Texas A&M
“The ideal bioink must be capable of being extruded into stable 3D structures while protecting cells during and after printing and providing an appropriate environment that can be remodeled into the target tissue,” Gaharwar said. “Unfortunately, conventional hydrogels are weak and poorly printable.”
“Unfortunately, conventional hydrogels are weak and poorly printable.”
The group’s NICE bioink formulation is used specifically to print 3D bone. NICE bioinks are a combination of two reinforcement techniques. Used together, they provide an effective reinforcement that results in much stronger bone structures. The NICE bioinks allow precise control over mechanical properties and degradation characteristics, enabling custom 3D fabrication of mechanically resilient, cellularized structures, Gaharwar said.
Further Reading: 3D Printing Organs Nearing Clinical Trials
Once the bioprinting process is complete, the cell-laden NICE networks are crosslinked to form stronger scaffolds. With the technique, the researchers have been able to produce full-scale, cell-friendly reconstructions of human body parts, including ears, blood vessels, cartilage, and bone segments.
This step toward 3D-printed replacements of human parts comes from a team at University of California, San Diego.
Most 3D bioprinting is done in culture dishes, but the UCSD team was able to do this in laboratory rats.
The scientists at UCSD printed out small implants made of softgel and then filled them with neural stem cells. Image: University of California, San Diego
The scientists first printed out small implants made of softgel, then filled them with neural stem cells, again using a printer. The implants were then surgically placed inside a tiny gap in a rat’s spinal cord. The precision 3D printing allowed the softgel and cellular matrix to fit accurately into the gap, or wound, said Shaochen Chen, a professor of nanoengineering at the university and a team leader.
Over time the new nerve cells and axons grew and formed new connections across the cut spinal cord of the animal. These nerve cells connected not only with one another but with the host spinal cord tissue and the circulatory systems of the rat. The lab-grown cells then successfully bridged the gap in the spinal cord and partially restored movement to the animal's hind quarters, said Mark Tuszinski, a professor of neuroscience at the university.
The lab-grown cells then successfully bridged the gap in the spinal cord and partially restored movement to the animal's hind quarters, said Mark Tuszinski, a professor of neuroscience at the university.
The researchers said that bioprinted tissue can be used to test the effects of drug treatments and, eventually achieve the 3D bioprinting goal: printing entire organs that can be grown and then transplanted into a patient.
Jean Thilmany is an independent writer in St. Paul, Minn.
3D printed organs have taken root
Subscribe to our ”Context” newsletter: it will help you understand the events.
Image copyright Wake Forest
Image captionAlmost any replica of a human organ can be made using 3D technology published in the journal Nature Biotechnology the results of the development of American scientists .
Breakthrough opening allows the use of living tissue to repair damaged organs.
A medical professor at University College London called the new technology "the goose that lays the golden eggs."
The idea of integrating individual human stem cells into a 3D printed replica of a damaged organ could revolutionize regenerative medicine.
Replacing a broken jaw, damaged heart muscle, or restoring a missing ear to a person is easy with this technology.
Today, the main problem of transplantation of artificially regenerated organs is the difficulty of maintaining their viability - tissues over 0.2 mm thick lack oxygen and nutrients.
Sponge
A team from the American medical center Wake Forest has developed a new technique that allows you to make a living tissue penetrated by microchannels using a 3D printer. The fabric has a sponge-like base, which allows nutrients and neural networks to penetrate into its structure.
Image copyright, Wake Forest
Image caption,An integrated regenerative system is used to 3D print body parts
The technology is an integrated system, part of which is responsible for tissue growth, the other is for making an exact copy of the replaced organ on a 3D printer .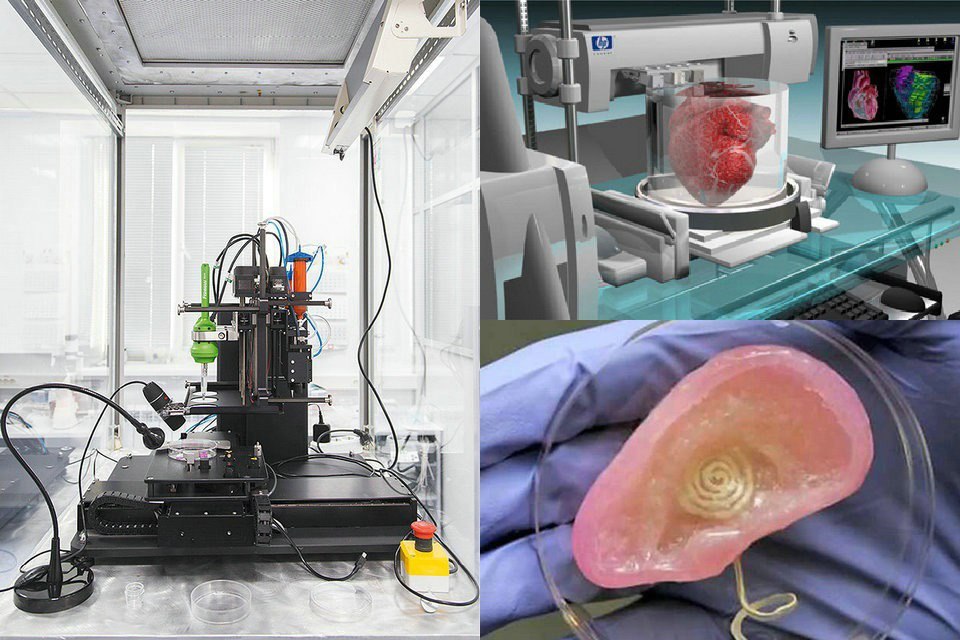
The starting material consists of a biodegradable plastic that forms the outer structure of the replicated organ and a water-based gel that contains the cells and stimulates their growth.
Animal testing has shown that after implantation, the plastic is gradually degraded and replaced by a natural structural matrix of proteins produced by the cells.
Blood vessels and nerves are rotated directly into the implants.
Powerful possibilities
According to Professor Anthony Atala, lead researcher at the Wake Forest Center, it is now possible to print human tissue, but scientists want to wait until animal tests are completed to understand how durable the recreated organs are.
Image copyright Wake Forest
Photo captionThis is what a broken jawbone looks like on a CT scan
Whatever the case, 3D printing opens up new possibilities for medicine.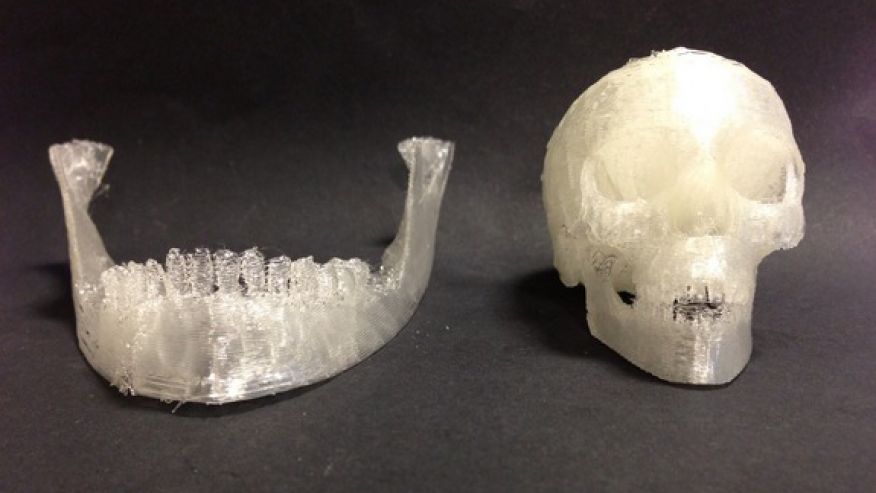 “Let’s say we have a patient with a jaw injury that is missing part of it. We do a tomography on the patient, then we send the data to the printer, and it will create the missing part of the jawbone that will completely fit the patient,” he told the BBC.
“Let’s say we have a patient with a jaw injury that is missing part of it. We do a tomography on the patient, then we send the data to the printer, and it will create the missing part of the jawbone that will completely fit the patient,” he told the BBC.
image copyrightWake Forest
Image caption,And so, the missing fragment made using a 3D printer
Technologies using biodegradable materials, which are then soaked in a solution with stem cells, are already being used.
Two years ago, Wake Forest Medical Center experimented with transplanting lab-grown female genital organs, but in general, the possibilities of such procedures are limited due to problems with maintaining cell viability.
According to Professor Atala, their recent experiment created a wide variety of tissues - muscles, soft cartilage and hard bones - which indicates the vast possibilities of the new technology.
Golden Goose
Professor Martin Birchall, a surgeon at University College London, says the results of the study are striking.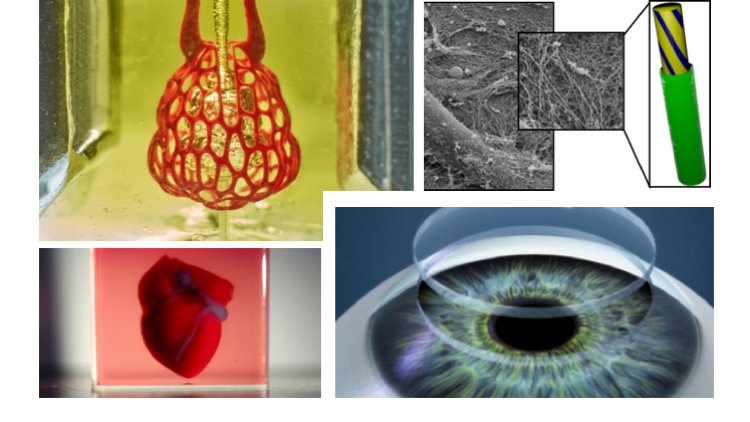
"The prospect of using 3D printing of human tissues and organs for implantation was real, but, I confess, I did not expect to see such rapid progress. What they managed to create can be called a goose that lays golden eggs!" - the doctor was delighted.
He also believes that more testing is needed before the new technology can be used in humans, but he hopes it will take a little time.
"Given the scale of this breakthrough research, advances in other areas, the resources available to Wake Forest scientists, and the pressing needs of public health, I believe that in less than a decade, surgeons like myself will be able to operate on printed organs. and fabrics. I can't wait," adds Martin Birchall.
Doctor prints knee cartilage on a 3D printer
Archive
0005
Dr. Daryll D'Lima and colleagues at the Scripps Clinic in La Jolla, California are working on a new 3D bioprinting technology that will allow living cartilage to be implanted into a patient's body, thereby providing better treatment for knee injuries.
Colleagues consider Dr. D'Lim a very resourceful person. He is a doctor and scientist who uses new technologies from various fields of science to help his patients.
Recently, Dr. D'Lima has been working on a technology to implant 3D-printed cartilage tissue into the patient's body to restore the structure of damaged knees. Cartilage is the tissue that connects the knee joints. It is known that it heals slowly and painfully.
Doctors are now advising patients to endure pain unless an artificial joint is needed. This procedure also causes severe pain and rarely solves the problem.
Hoping to find an alternative to conventional joint implants, Dr. D'Lima and his team decided to make a 3D bioprinter out of an old HP inkjet printer and print living cartilage tissue on it. The 3D printer sprays a mixture of cartilage progenitor cells and a liquid that hardens under ultraviolet light. Bone cells are also printed on it, which are placed where the cartilage connects to the bone. The volume of a drop of liquid with living cells is 1 picoliter, which is quite enough to correct even microscopic disorders of the patient's bone or cartilage.
The volume of a drop of liquid with living cells is 1 picoliter, which is quite enough to correct even microscopic disorders of the patient's bone or cartilage.
Dr. D'Lima compares the process of grafting cartilage into the knee to filling in potholes: “You automatically fill in the depressions as you print. You get cartilage that is perfectly shaped to the existing tissue, which we have never been able to do with conventional implants,” said D’Lima. Printing on a pre-existing joint provides a better fit for new and old cartilage than grafting lab-grown cartilage that has to be trimmed to fit better. Another advantage of cartilage printing is that there is no longer a need to postpone the operation for later - it can be done right away.
People born during the "baby boom" age, and the problem of excess weight is becoming more and more aggravated. This means that in the future, knee injuries will become an even more serious cause for concern. According to a GlobalData report, the knee implant market will grow by 6. 8% annually from 2010 to 2017.
8% annually from 2010 to 2017.
The research of Dr. D'Lim and his team has been recognized by several institutes. He received a $3.1 million grant from the California Institute for Regenerative Medicine Stem Cell Research Agency to research the use of natural and artificial embryonic stem cells in cartilage growth.
D'Lima believes that it will take at least a year to create a full-fledged 3D bioprinter. He is currently conducting animal experiments to prove the promise of his idea, which is sponsored by the George Schaeffer Family Foundation.
Similar 3D bioprinting inventions are popping up around the world. Just last month, a prototype 3D printed BioPen was handed over to researchers at St Vincent's Hospital in Melbourne, and Organovo created the world's first 3D printed liver tissue on a printer in its labs.
However, the printing of living tissue is still a very difficult task. For example, to print muscle tissue, you need to synthesize different types of cells and a whole network of blood vessels.