3D printed hydrogels
3D printing of high-strength chitosan hydrogel scaffolds without any organic solvents
Luyu Zhou, ‡ab Hamed Ramezani,‡ab Miao Sun,c Mingjun Xie,ab Jing Nie,ab Shang Lv,ab Jie Cai, d Jianzhong Fu*ab and Yong He *ab
Author affiliations
* Corresponding authors
a
State Key Laboratory of Fluid Power and Mechatronic Systems, School of Mechanical Engineering, Zhejiang University, Hangzhou 310027, China
E-mail:
fjz@zju. edu.cn, [email protected]
b Key Laboratory of 3D Printing Process and Equipment of Zhejiang Province, School of Mechanical Engineering, Zhejiang University, Hangzhou 310027, China
c Department of Oral and Maxillofacial Surgery, Affiliated Stomatology Hospital, School of Medicine, Zhejiang University, Hangzhou 310000, China
d College of Chemistry & Molecular Sciences, Wuhan University, Wuhan 430072, China
Abstract
3D printing of chitosan hydrogels has attracted wide interest because of their excellent biocompatibility, antibacterial activities, biodegradability, zero toxicity and low cost. However, chitosan inks are often involved in toxic and organic solvents. Moreover, the recently reported 3D-printed chitosan scaffolds lack enough strength, thus limiting their use in tissue engineering. Here, we reported a chitosan ink obtained by dissolving chitosan into an alkali aqueous solution. This chitosan ink is a stable solution at low temperature (5 °C), but once heated, the chitosan chains self-assemble to lead to gelation. Based on this principle, a corresponding direct ink writing (DIW) method was developed to print high-strength chitosan hydrogels. Specifically, the chitosan ink was extruded into heated deionized water to complete the in situ gelation. The temperature of the nozzle and hot water was well controlled to keep the printing process stable. The rheological behavior of the chitosan ink was investigated and the printing parameters were systematically studied to print chitosan hydrogel scaffolds with high quality and high strength.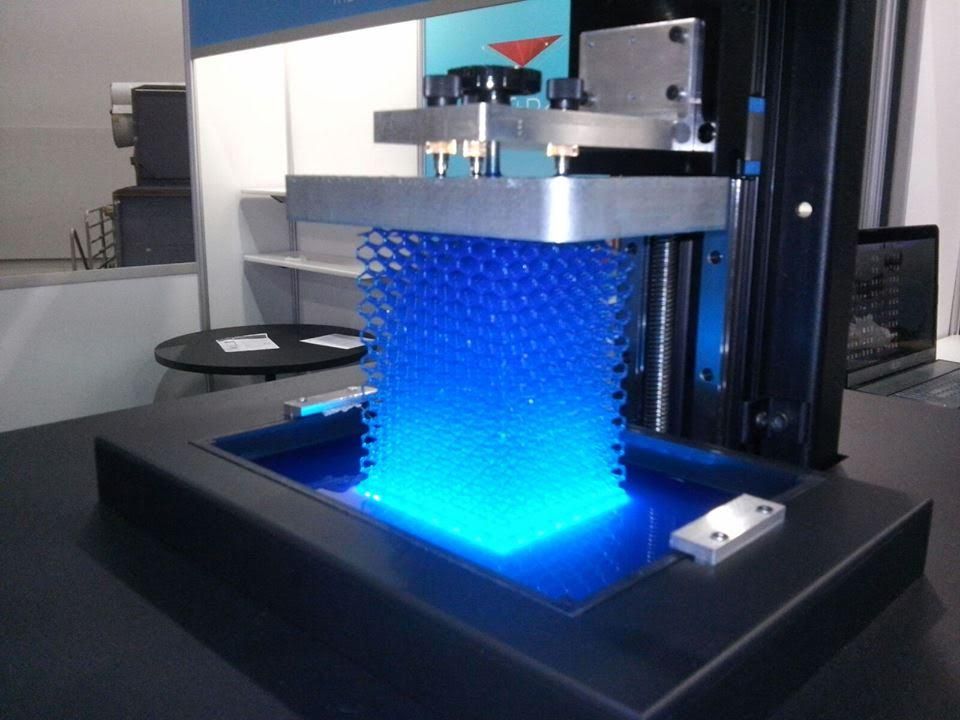 Based on these, high-strength (2.31 MPa for compressive strength) and complex chitosan hydrogel structures can be directly printed. The cell culture and the wound healing results further show that the printed chitosan scaffolds with this method have great potential in tissue engineering.
Based on these, high-strength (2.31 MPa for compressive strength) and complex chitosan hydrogel structures can be directly printed. The cell culture and the wound healing results further show that the printed chitosan scaffolds with this method have great potential in tissue engineering.
3D printing of hydrogel composite systems: Recent advances in technology for tissue engineering
1. Wang X, Jiang M, Zhou Z W, et al. 3D printing of polymer matrix composites:A review and prospective. Compos B Eng. 2017;110:442–458. http://dx.doi.org/10.1016/j.compositesb.2016.11.034. [Google Scholar]
2. Chua C K, Leong K F. 3D printing and additive manufacturing :Principles and applications. 4th ed. Singapore: World Scientific Publishing; 2015. [Google Scholar]
3. Billiet T, Vandenhaute M, Schelfhout J, et al. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 2012;33(26):6020–6041. http://dx.doi.org/10.1016/j.biomaterials.2012.04.050. [PubMed] [Google Scholar]
Biomaterials. 2012;33(26):6020–6041. http://dx.doi.org/10.1016/j.biomaterials.2012.04.050. [PubMed] [Google Scholar]
4. Ballyns J J, Gleghorn J P, Niebrzydowski V, et al. Image-guided tissue engineering of anatomically shaped implants via MRI and micro-CT using injection molding. Tissue Eng Part A. 2008;14(7):1195–1202. http://dx.doi.org/10.1089/ten.tea.2007.0186. [PubMed] [Google Scholar]
5. Chia H N, Wu B M. Recent advances in 3D printing of biomaterials. J Biol Eng. 2015;9(1):4. http://dx.doi.org/10.1186/S13036-015-0001-4. [PMC free article] [PubMed] [Google Scholar]
6. Seyednejad H, Gawlitta D, Kuiper R V, et al. In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxyl-functionalized poly(epsiloncaprolactone) Biomaterials. 2012;33(17):4309–4318. http://dx.doi.org/10.1016/j.biomaterials.2012.03.002. [PubMed] [Google Scholar]
7. Wu G H, Hsu S H. Review:Polymeric-Based 3D printing for tissue engineering. J Med Bioeng. 2015;35(3):285292. http://dx.doi.org/10.1007/s40846-015-0038-3. [PMC free article] [PubMed] [Google Scholar]
2015;35(3):285292. http://dx.doi.org/10.1007/s40846-015-0038-3. [PMC free article] [PubMed] [Google Scholar]
8. Utech S, Boccaccini A R. A review of hydrogelbased composites for biomedical applications:Enhancement of hydrogel properties by addition of rigid inorganic fillers. J Mater Sci. 2016;51(1):271–310. http://dx.doi.org/10.1007/s10853-015-9382-5. [Google Scholar]
9. Gaharwar A K, Peppas N A, Khademhosseini A. Nanocomposite hydrogels for biomedical applications. Biotechnol Bioeng. 2014;111(3):441–453. http://dx.doi.org/10.1002/bit.25160. [PMC free article] [PubMed] [Google Scholar]
10. Xu K, Wang J H, Chen Q, et al. Spontaneous volume transition of polyampholyte nanocomposite hydrogels based on pure electrostatic interaction. J Colloid Interface Sci. 2008;321(2):272–278. http://dx.doi.org/10.1016/jjcis.2008.02.024. [PubMed] [Google Scholar]
11. Kabiri K, Omidian H, Zohuriaan-Mehr M J, et al. Superabsorbent hydrogel composites and nanocomposites:A review. Polym Compos. 2011;32(2):277–289. http://dx.doi.org/10.1002/pc.21046. [Google Scholar]
2011;32(2):277–289. http://dx.doi.org/10.1002/pc.21046. [Google Scholar]
12. Thoniyot P, Tan M J, Karim A A, et al. NanoparticleHydrogel composites:Concept, design, and applications of these promising, multi-functional materials. Adv Sci. 2015;2(1-2) http://dx.doi.org/10.1002/Advs.201400010. [PMC free article] [PubMed] [Google Scholar]
13. Lee J W, Kim S Y, Kim S S, et al. Synthesis and characteristics of interpenetrating polymer network hydrogel composed of chitosan and poly(acrylic acid) J Appl Polym Sci. 1999;73(1):113–120. http://dx.doi.org/10.1002/(SICI)1097-4628(19990705)73:1<113::AID-APP13>3.0.C0;2-D. [Google Scholar]
14. Ehrburger P, Donnet J B. Interface in compositematerials. Philos Trans A Math Phys Eng Sci. 1980;294(1411):495–505. http://dx.doi.org/10.1098/rsta.1980.0059. [Google Scholar]
15. Jeong S H, Koh Y H, Kim S W, et al. Strong and biostable hyaluronic acid-calcium phosphate nanocomposite hydrogel via in situ precipitation process. Biomacromolecules. 2016;17(3):841–851. http://dx.doi.org/10.1021/acs.biomac.5b01557. [PubMed] [Google Scholar]
Biomacromolecules. 2016;17(3):841–851. http://dx.doi.org/10.1021/acs.biomac.5b01557. [PubMed] [Google Scholar]
16. Wust S, Godla M E, Muller R, et al. Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomater. 2014;10(2):630–640. http://dx.doi.org/10.1016/j.actbio.2013.10.016. [PubMed] [Google Scholar]
17. Duan B, Hockaday L A, Kang K H, et al. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A. 2013;101(5):1255–1264. http://dx.doi.org/10.1002/jbm.a.34420. [PMC free article] [PubMed] [Google Scholar]
18. Melchels F P W, Feijen J, Grijpma D W. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31(24):6121–6130. http://dx.doi.org/10.1016/j.biomaterials.2010.04.050. [PubMed] [Google Scholar]
19. Bertsch A, Jiguet S, Bernhard P, et al. Microstereolithography A review. Rapid Prototyping Technologies. 2003;758:3–15. [Google Scholar]
Rapid Prototyping Technologies. 2003;758:3–15. [Google Scholar]
20. Beluze L, Bertsch A, Renaud P. Microstereolithography:A new process to build complex 3D objects Design. Test, and Microfabrication of Mems and Moems, Pts 1 and. 1999;2;3680:808–817. http://dx.doi.org/10.1117/12.341277. [Google Scholar]
21. Choi J S, Kang H W, Lee I H, et al. Development of micro-stereolithography technology using a UV lamp and optical fiber. Int J Adv Manuf Technol. 2009;41(3-4):281–286. http://dx.doi.org/10.1007/s00170-008-1461-1. [Google Scholar]
22. Bertsch A, Renaud P, Vogt C, et al. Rapid prototyping of small size objects. Rapid Prototyp J. 2000;6(4):259–266. http://dx.doi.org/10.1108/13552540010373362. [Google Scholar]
23. Sun C, Fang N, Wu D M, et al. Projection microstereolithography using digital micro-mirror dynamic mask. Sens Actuators A Phys. 2005;121(1):113–120. http://dx.doi.org/10.1016/j.sna.2004.12.011. [Google Scholar]
24. Ambrosio L. Biomedical composites. 2nd ed. UK: Woodhead Publishing; 2010. [Google Scholar]
2nd ed. UK: Woodhead Publishing; 2010. [Google Scholar]
25. Maruo Sand Ikuta K. Submicron stereolithography for the production of freely movable mechanisms by using singlephoton polymerization. Sens Actuators A Phys. 2002;100(1):70–76. http://dx.doi.org/10.1016/S0924-4247(02)00043-2. [Google Scholar]
26. Lee K S, Kim R H, Yang D Y, et al. Advances in 3D nano/microfabrication using two-photon initiated polymerization. Prog Polym Sci. 2008;33(6):631–681. http://dx.doi.org/10.1016/j.progpolymsci.2008.01.001. [Google Scholar]
27. Weiss T, Hildebrand G, Schade R, et al. Two-Photon polymerization for microfabrication of three-dimensional scaffolds for tissue engineering application. Eng Life Sci. 2009;9(5):384–390. http://dx.doi.org/10.1002/elsc.200900002. [Google Scholar]
28. Ostendorf A, Chichkov B N. Two-photon polymerization:A new approach to micromachining. Photonics Spectra. 2006;40(10):72–80. [Google Scholar]
29. Hutmacher D W, Sittinger M, Risbud M V. Scaffold-based tissue engineering:Rationale for computeraided design and solid free-form fabrication systems. Trends Biotechnol. 2004;22(7):354–362. http://dx.doi.org/10.1016/j.tibtech.2004.05.006. [PubMed] [Google Scholar]
Trends Biotechnol. 2004;22(7):354–362. http://dx.doi.org/10.1016/j.tibtech.2004.05.006. [PubMed] [Google Scholar]
30. Bikas H, Stavropoulos P, Chryssolouris G. Additive manufacturing methods and modelling approaches:A critical review. Int J Adv Manuf Technol. 2016;83(1-4):389–405. http://dx.doi.org/10.1007/s00170-015-7576-2. [Google Scholar]
31. Anitha R, Arunachalam S, Radhakrishnan P. Critical parameters influencing the quality of prototypes in fused deposition modelling. J Mater Process Technol. 2001;118(1):385–388. http://dx.doi.org/10.1016/S0924-0136(01)00980-3. [Google Scholar]
32. Xiong Z, Yan Y, Zhang R, et al. Fabrication of porous poly (L-lactic acid) scaffolds for bone tissue engineering via precise extrusion. Scr Mater. 2001;45(7):773–779. http://dx.doi.org/10.1016/S1359-6462(01)01094-6. [Google Scholar]
33. Greulich M, Greul M, Pintat T. Fast, functional prototypes via multiphase jet solidification. Rapid Prototyp J. 1995;1(1):20–25. http://dx.doi.org/10. 1108/13552549510146649. [Google Scholar]
1108/13552549510146649. [Google Scholar]
34. Shor L, Güçeri S, Chang R, et al. Precision extruding deposition (PED) fabrication of polycaprolactone (PCL) scaffolds for bone tissue engineering. Biofabrication. 2009;1(1):015003. http://dx.doi.org/10.1088/1758-5082/1Z1/015003. [PubMed] [Google Scholar]
35. Torres J, Cotelo J, Karl J, et al. Mechanical property optimization of FDM PLA in shear with multiple objectives. JOM. 2015;67(5):1183–1193. http://dx.doi.org/10.1007/s11837-015-1367-y. [Google Scholar]
36. Yeong W Y, Chua C K, Leong K F, et al. Rapid prototyping in tissue engineering:Challenges and potential. Trends Biotechnol. 2004;22(12):643–652. http://dx.doi.org/10.1016/j.tibtech.2004.10.004. [PubMed] [Google Scholar]
37. Gates R D, Baghdasarian G, Muscatine L. Temperature stress causes host-cell detachment in symbiotic cnidarians-implications for coral bleaching. Biol Bull. 1992;182(3):324–332. http://dx.doi.org/10.2307/1542252. [PubMed] [Google Scholar]
38. Landers R, Mülhaupt R. Desktop manufacturing of complex objects, prototypes and biomedical scaffolds by means of computer-assisted design combined with computer-guided 3D plotting of polymers and reactive oligomers. Macromol Mater Eng. 2000;282(1):17–21. http://dx.doi.org/10.1002/1439-2054(20001001)282:1<17::AIDMAME17>3.0.CO;2-8. [Google Scholar]
Landers R, Mülhaupt R. Desktop manufacturing of complex objects, prototypes and biomedical scaffolds by means of computer-assisted design combined with computer-guided 3D plotting of polymers and reactive oligomers. Macromol Mater Eng. 2000;282(1):17–21. http://dx.doi.org/10.1002/1439-2054(20001001)282:1<17::AIDMAME17>3.0.CO;2-8. [Google Scholar]
39. Billiet T, Gevaert E, De Schryver T, et al. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials. 2014;35(1):49–62. http://dx.doi.org/10.1016/j.biomaterials.2013.09.078. [PubMed] [Google Scholar]
40. Luo Y, Lode A, Akkineni A R, et al. Concentrated gelatin/alginate composites for fabrication of predesigned scaffolds with a favorable cell response by 3D plotting. RSC Adv. 2015;5(54):43480–43488. http://dx.doi.org/10.1039/C5RA04308E. [Google Scholar]
41. Akkineni A R, Luo Y, Schumacher M, et al. 3D plotting of growth factor loaded calcium phosphate cement scaffolds. Acta Biomater. 2015;27:264–274. http://dx.doi.org/10.1016/j.actbio.2015.08.036. [PubMed] [Google Scholar]
Acta Biomater. 2015;27:264–274. http://dx.doi.org/10.1016/j.actbio.2015.08.036. [PubMed] [Google Scholar]
42. Yilgor P, Sousa R A, Reis R L, et al. 3D plotted PCL scaffolds for stem cell based bone tissue engineering, Macromol Symp, 2008. Wiley Online Library. 269:92–99. http://dx.doi.org/10.1002/masy.200850911. [Google Scholar]
43. Landers R, Mulhaupt R. Desktop manufacturing of complex objects, prototypes and biomedical scaffolds by means of computer-assisted design combined with computer-guided 3D plotting of polymers and reactive oligomers. Macromol Mater Eng. 2000;282(9):17–21. http://dx.doi.org/10.1002/1439-2054(20001001)282:1<17::AidMame17>3.0.Co;2-8. [Google Scholar]
44. Smay J E, Gratson G M, Shepherd R F, et al. Directed colloidal assembly of 3D periodic structures. Adv Mater. 2002;14(18):1279–1283. [Google Scholar]
45. Ahn B Y, Duoss E B, Motala M J, et al. Omnidirectional printing of flexible, stretchable, and spanning silver microelectrodes. Science. 2009;323(5921):1590–1593. https:// dx.doi.org/10.1126/science.1168375. [PubMed] [Google Scholar]
2009;323(5921):1590–1593. https:// dx.doi.org/10.1126/science.1168375. [PubMed] [Google Scholar]
46. Vozzi G, Previti A, De Rossi D, et al. Microsyringebased deposition of two-dimensional and three-dimensional polymer scaffolds with a well-defined geometry for application to tissue engineering. Tissue Eng. 2002;8(6):10891098. https://dx.doi.org/10.1089/107632702320934182. [PubMed] [Google Scholar]
47. Tartarisco G, Gallone G, Carpi F, et al. Polyurethane unimorph bender microfabricated with pressure assisted microsyringe (PAM) for biomedical applications. Mater Sci Eng C Mater Biol Appl. 2009;29(6):1835–1841. https://dx.doi. org/10.1016/j.msec.2009.02.017. [Google Scholar]
48. Xiong Z, Yan Y, Wang S, et al. Fabrication of porous scaffolds for bone tissue engineering via low-temperature deposition. Scr Mater. 2002;46(11):771–776. https://dx.doi. org/10.1016/S1359-6462(02)00071-4. [Google Scholar]
49. Liu L, Xiong Z, Zhang R, et al. A novel osteochondral scaffold fabricated via multi-nozzle low-temperature deposition manufacturing. J Bioact Compat Polym. 2009;24(1):18–30. https://dx.doi.org/10.1177/0883911509102347. [Google Scholar]
J Bioact Compat Polym. 2009;24(1):18–30. https://dx.doi.org/10.1177/0883911509102347. [Google Scholar]
50. Vadnere M, Amidon G, Lindenbaum S, et al. Thermodynamic studies on the gel-sol transition of some pluronic polyols. Int J Pharm. 1984;22(2-3):207–218. https:// dx.doi.org/10.1016/0378-5173(84)90022-X. [Google Scholar]
51. Kim J Y, Cho D-W. Blended PCL/PLGA scaffold fabrication using multi-head deposition system. Microelectron Eng. 2009;86(4):1447–1450. https://dx.doi.org/10.1016/ j.mee.2008.11.026. [Google Scholar]
52. Domingos M, Dinucci D, Cometa S, et al. Polycaprolactone scaffolds fabricated via bioextrusion for tissue engineering applications. Int J Biomater. 2009;2009(1687-8787):239643. https:// dx.doi.org/10.1155/2009/239643. [PMC free article] [PubMed] [Google Scholar]
53. Lam C, Olkowski R, Swieszkowski W, et al. Mechanical and in vitro evaluations of composite PLDLLA/TCP scaffolds for bone engineering. Virtual Phys Prototyp. 2008;3(4):193–197. ttps://dx.doi.org/10.1080/17452750802551298. [Google Scholar]
ttps://dx.doi.org/10.1080/17452750802551298. [Google Scholar]
54. Lim T, Bang C, Chian K, et al. Development of cryogenic prototyping for tissue engineering. Virtual Phys Prototyp. 2008;3(1):25–31. https://dx.doi.org/10.1080/17452750701799303. [Google Scholar]
55. Bang Pham C, Fai Leong K, Chiun Lim T, et al. Rapid freeze prototyping technique in bio-plotters for tissue scaffold fabrication. Rapid Prototyp J. 2008;14(4):246–253. https://dx.doi.org/10.1108/13552540810896193. [Google Scholar]
56. Lu L, Zhang Q, Wootton D, et al. A novel sucrose porogen-based solid freeform fabrication system for bone scaffold manufacturing. Rapid Prototyp J. 2010;16(5):365–376. https://dx.doi.org/10.1108/13552541011065768. [Google Scholar]
57. Cima M, Sachs E, Fan T, et al. Three-dimensional printing techniques. US patent 53∢0, 1995 July 2 [Google Scholar]
58. Mei J, Lovell M, Rand Mickle M H. Formulation and processing of novel conductive solution inks in continuous inkjet printing of 3-D electric circuits. IEEE Trans Compon Packaging Manuf Technol. 2005;28(3):265–273. https://dx.doi. org/10.1109/TEPM.2005.852542. [Google Scholar]
IEEE Trans Compon Packaging Manuf Technol. 2005;28(3):265–273. https://dx.doi. org/10.1109/TEPM.2005.852542. [Google Scholar]
59. Saunders R E, Gough J E, Derby B. Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing. Biomaterials. 2008;29(2):193–203. https://dx.doi. org/10.1016/j.biomaterials.2007.09.032. [PubMed] [Google Scholar]
60. Nakamura M, Kobayashi A, Takagi F, et al. Biocompatible inkjet printing technique for designed seeding of individual living cells. Tissue Eng. 2005;11(11-12):1658–1666. https://dx.doi. org/10.1089/ten.2005.11.1658. [PubMed] [Google Scholar]
61. Cui X, Dean D, Ruggeri Z M, et al. Cell damage evaluation of thermal inkjet printed Chinese hamster ovary cells. Biotechnol Bioeng. 2010;106(6):963–969. https://dx.doi. org/10.1002/bit.22762. [PubMed] [Google Scholar]
62. Leukers B, Gülkan H, Irsen S H, et al. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J MATER SCI-MATER M. 2005;16(12):1121–1124. https://dx.doi. org/10.1007/s10856-005-4716-5. [PubMed] [Google Scholar]
https://dx.doi. org/10.1007/s10856-005-4716-5. [PubMed] [Google Scholar]
63. Inzana J A, Olvera D, Fuller S M, et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014;35(13):4026–4034. https:// dx.doi.org/10.1016/j.biomaterials.2014.01.064. [PMC free article] [PubMed] [Google Scholar]
64. Levy A, Miriyev A, Elliott A, et al. Additive manufacturing of complex-shaped graded TiC/steel composites. Mater Design. 2017;118:198–203. https://dx.doi.org/10.1016/j.matdes.2017.01.024. [Google Scholar]
65. Pfister A, Landers R, Laib A, et al. Biofunctional rapid prototyping for tissue-engineering applications:3D bioplotting versus 3D printing. J Polym Sci Pol Chem. 2004;42(3):624–638. https:// dx.doi.org/10.1002/pola.10807. [Google Scholar]
66. Boland T, Tao X, Damon B J, et al. Drop-on-demand printing of cells and materials for designer tissue constructs. Mater Sci Eng C Mater Biol Appl. 2007;27(3):372–376. https:// dx. doi.org/10.1016/j.msec.2006.05.047. [Google Scholar]
doi.org/10.1016/j.msec.2006.05.047. [Google Scholar]
67. Sun J, Ng J H, Fuh Y H, et al. Comparison of micro-dispensing performance between micro-valve and piezoelectric printhead. Microsyst Technol. 2009;15(9):14371448. https:// dx.doi.org/10.1007/s00542-009-0905-3. [Google Scholar]
68. Zustiak S P, Leach J B. Hydrolytically degradable poly (ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules. 2010;11(5):1348–1357. https:// dx.doi.org/10.1021/bm100137q. [PMC free article] [PubMed] [Google Scholar]
69. Killion J A, Geever L M, Devine D M, et al. Compressive strength and bioactivity properties of photopolymerizable hybrid composite hydrogels for bone tissue engineering. Int J Polym Mater Po. 2014;63(13):641–650. https:// dx.doi.org/10.1080/00914037. 2013.⅞38. [Google Scholar]
70. Bakarich S E, Gorkin R, Gately R, et al. 3D printing of tough hydrogel composites with spatially varying materials properties. Addit Manuf. 2017;14:24–30. https:// dx.doi. org/10.1016/j.addma.2016.12.003. [Google Scholar]
2017;14:24–30. https:// dx.doi. org/10.1016/j.addma.2016.12.003. [Google Scholar]
71. Zhao L, Lee V K, Yoo S-S, et al. The integration of 3-D cell printing and mesoscopic fluorescence molecular tomography of vascular constructs within thick hydrogel scaffolds. Biomaterials. 2012;33(21):5325–5332. http://dx.doi.org/10.1016/j.biomaterials.2012.04.004. [PMC free article] [PubMed] [Google Scholar]
72. Hong S, Sycks D, Chan H F, et al. 3D printing of highly stretchable and tough hydrogels into complex, cellularized structures. Adv Mater. 2015;27(27):4035–4040. http://dx.doi.org/10.1002/adma.201501099. [PMC free article] [PubMed] [Google Scholar]
73. Markstedt K, Mantas A, Tournier I, et al. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules. 2015;16(5):1489–1496. http://dx.doi.org/10.1021/acs.biomac.5b00188. [PubMed] [Google Scholar]
74. Rutz A L, Hyland K E, Jakus A E, et al. A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels. AdvMater. 2015;27(9):1607–1614. http://dx.doi.org/10.1002/adma.201405076. [PMC free article] [PubMed] [Google Scholar]
AdvMater. 2015;27(9):1607–1614. http://dx.doi.org/10.1002/adma.201405076. [PMC free article] [PubMed] [Google Scholar]
75. Xu M, Wang X, Yan Y, et al. An cell-assembly derived physiological 3D model of the metabolic syndrome, based on adipose-derived stromal cells and a gelatin/alginate/fibrinogen matrix. Biomaterials. 2010;31(14):3868–3877. http://dx.doi.org/10.1016/j.biomaterials.2010.01.111. [PubMed] [Google Scholar]
76. Akkineni A R, Ahlfeld T, Funk A, et al. Highly concentrated alginate-gellan gum composites for 3D plotting of complex tissue engineering scaffolds. Polymers. 2016;8(5):170. http://dx.doi.org/10.3390/polym8050170. [PMC free article] [PubMed] [Google Scholar]
77. Boere K W, Blokzijl M M, Visser J, et al. Biofabrication of reinforced 3D-scaffolds using two-component hydrogels. J Mater Chem B Mater Biol Med. 2015;3(46):9067–9078. http://dx.doi.org/10.1039/C5TB01645B. [PMC free article] [PubMed] [Google Scholar]
78. Censi R, Schuurman W, Malda J, et al. A printable photopolymerizable thermosensitive p (HPMAm-lactate)PEG hydrogel for tissue engineering. Adv Funct Mater. 2011;21(10):1833–1842. http://dx.doi.org/10.1002/adfm.201002428. [Google Scholar]
A printable photopolymerizable thermosensitive p (HPMAm-lactate)PEG hydrogel for tissue engineering. Adv Funct Mater. 2011;21(10):1833–1842. http://dx.doi.org/10.1002/adfm.201002428. [Google Scholar]
79. Osterbur L. 3D printing of hyaluronic acid scaffolds for tissue engineering applications [Internet] 2013 Available from: http://hdl.handle.net/2142/44207 .
80. Wang X, Cui T, Yan Y, et al. Peroneal nerve regeneration using a unique bilayer polyurethane-collagen guide conduit. J Bioact Compat Polym. 2009;24(2):109–127. http://dx.doi.org/10.1177/0883911508101183. [Google Scholar]
81. Mogas-Soldevila L, Duro-Royo J, Oxman N. Water-based robotic fabrication:Large-Scale additive manufacturing of functionally graded hydrogel composites via multichamber extrusion. 3D Print Addit Manuf. 2014;1(3):141–151. http://dx.doi.org/10.1089/3dp.2014.0014. [Google Scholar]
82. Shie M-Y, Chang W-C, Wei L-J, et al. 3D printing of cytocompatible water-based light-cured polyurethane with hyaluronic acid for cartilage tissue engineering applications. Materials. 2017;10(2):136. http://dx.doi.org/10.3390/ ma10020136. [PMC free article] [PubMed] [Google Scholar]
Materials. 2017;10(2):136. http://dx.doi.org/10.3390/ ma10020136. [PMC free article] [PubMed] [Google Scholar]
83. Wang X H, Tolba E, Schroder H C, et al. Effect of bioglass on growth and biomineralization of Saos-2 cells in hydrogel after 3D cell bioprinting. Plos One. 2014;9(11):e112497. http://dx.doi.org/10.1371/journal.pone.0112497. [PMC free article] [PubMed] [Google Scholar]
84. Sayyar S, Gambhir S, Chung J, et al. 3D printable conducting hydrogels containing chemically converted graphene. Nanoscale. 2017;9(5):2038–2050. http://dx.doi.org/10.1039/c6nr07516a. [PubMed] [Google Scholar]
85. Demirtas T T, Irmak G, Gumusderelioglu M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication. 2017;9(3):035003. http://dx.doi.org/10.1088/1758-5090/Aa7b1d. [PubMed] [Google Scholar]
86. Skardal A, Zhang J X, McCoard L, et al. Dynamically crosslinked gold nanoparticle-Hyaluronan hydrogels. Adv Mater. 2010;22(42):4736. http://dx.doi.org/10.1002/adma. 201001436. [PubMed] [Google Scholar]
201001436. [PubMed] [Google Scholar]
87. Fedorovich N E, Wijnberg H M, Dhert W J, et al. Distinct tissue formation by heterogeneous printing of osteoand endothelial progenitor cells. Tissue Eng Part A. 2011;17(15-16):2113–2121. http://dx.doi.org/10.1089/ten.tea.2011.0019. [PubMed] [Google Scholar]
88. Panhuis M I H, Heurtematte A, Small W R, et al. Inkjet printed water sensitive transparent films from natural gum-carbon nanotube composites. Soft Matter. 2007;3(7):840843. http://dx.doi.org/10.1039/b704368f. [PubMed] [Google Scholar]
89. Heo D N, Castro N J, Lee S J, et al. Enhanced bone tissue regeneration using a 3D printed microstructure incorporated with a hybrid nano hydrogel. Nanoscale. 2017;9(16):5055–5062. http://dx.doi.org/10.1039/c6nr09652b. [PMC free article] [PubMed] [Google Scholar]
90. Zhu W, Holmes B, Glazer R I, et al. 3D printed nanocomposite matrix for the study of breast cancer bone metastasis. Nanomedicine. 2016;12(1):69–79. http://dx.doi.org/10.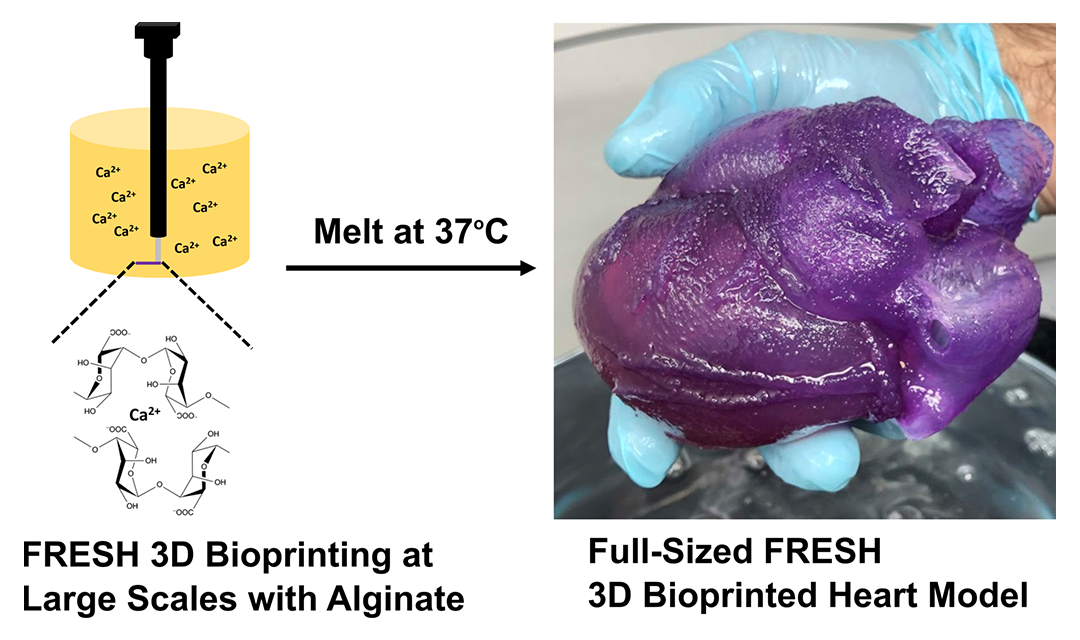 1016/j.nano.2015.09.010. [PubMed] [Google Scholar]
1016/j.nano.2015.09.010. [PubMed] [Google Scholar]
91. Castro N J, O'Brien J, Zhang L G. Integrating biologically inspired nanomaterials and table-top stereolithography for 3D printed biomimetic osteochondral scaffolds. Nanoscale. 2015;7(33):14010–14022. http://dx.doi.org/10.1039/c5nr03425f. [PMC free article] [PubMed] [Google Scholar]
92. Gladman A S, Matsumoto E A, Nuzzo R G, et al. Biomimetic 4D printing. Nat Mater. 2016;15(4):413–418. http://dx.doi.org/10.1038/NMAT4544. [PubMed] [Google Scholar]
93. Narayanan L K, Huebner P, Fisher M B, et al. 3D-Bioprinting of polylactic acid (PLA) nanofiber-alginate hydrogel bioink containing human adipose-derived stem cells. ACS Biomater Sci Eng. 2016;2(10):1732–1742. http://dx.doi.org/10.1021/acsbiomaterials.6b00196. [Google Scholar]
94. Agrawal A, Rahbar N, Calvert P D. Strong fiberreinforced hydrogel. Acta Biomaterialia. 2013;9(2):5313–5318. http://dx.doi.org/10.1016/j.actbio.2012.10.011. [PubMed] [Google Scholar]
95. Bakarich S E, Gorkin R, Panhuis M I H, et al. Threedimensional printing fiber reinforced hydrogel composites. ACS Appl Mater Interfaces. 2014;6(18):15998–16006. http://dx.doi.org/10.1021/am503878d. [PubMed] [Google Scholar]
Bakarich S E, Gorkin R, Panhuis M I H, et al. Threedimensional printing fiber reinforced hydrogel composites. ACS Appl Mater Interfaces. 2014;6(18):15998–16006. http://dx.doi.org/10.1021/am503878d. [PubMed] [Google Scholar]
96. Jin Y, Liu C, Chai W, et al. Self-Supporting nanoclay as internal scaffold mterial for direct printing of soft hydrogelcomposite structures in air. ACS Appl Mater Interfaces. 2017;9(20):17456–17465. http://dx.doi.org/10.1021/acsami.7b03613. [PubMed] [Google Scholar]
97. Zhai X, Ma Y, Hou C, et al. 3D-printed high strength bioactive supramolecular polymer/clay nanocomposite hydrogel scaffold for bone regeneration. ACS Biomater Sci Eng. 2017;3(6):1109–1118. http://dx.doi.org/10.1021/acsbiomaterials.7b00224. [Google Scholar]
98. Ahlfeld T, Cidonio G, Kilian D, et al. Development of a clay based bioink for 3D cell printing for skeletal application. Biofabrication. 2017;9(3) http://dx.doi.org/10.1088/1758-5090/aa7e96. [PubMed] [Google Scholar]
99. Egorov A A, Fedotov A Y, Mironov A V, et al. 3D printing of mineral-polymer bone substitutes based on sodium alginate and calcium phosphate. Beilstein J Nanotechnol. 2016;7(1):1794–1799. http://dx.doi.org/10.3762/bjnano.7.172. [PMC free article] [PubMed] [Google Scholar]
Egorov A A, Fedotov A Y, Mironov A V, et al. 3D printing of mineral-polymer bone substitutes based on sodium alginate and calcium phosphate. Beilstein J Nanotechnol. 2016;7(1):1794–1799. http://dx.doi.org/10.3762/bjnano.7.172. [PMC free article] [PubMed] [Google Scholar]
100. Rawat K, Agarwal S, Tyagi A, et al. Aspect ratio dependent cytotoxicity and antimicrobial properties of nanoclay. ApplBiochem Biotechnol. 2014;174(3):936–944. http://dx.doi.org/10.1007/s12010-014-0983-2. [PubMed] [Google Scholar]
101. Mourchid A, Delville A, Lambard J, et al. Phase diagram of colloidal dispersions of anisotropic charged particles:Equilibrium properties, structure, and rheology of laponite suspensions. Langmuir. 1995;11(6):1942–1950. http://dx.doi.org/10.1021/la00006a020. [Google Scholar]
102. Su D, Jiang L, Chen X, et al. Enhancing the gelation and bioactivity of injectable silk fibroin hydrogel with laponite nanoplatelets. ACS Appl Mater Interfaces. 2016;8(15):9619–9628. http://dx. doi.org/10.1021/acsami.6b00891. [PubMed] [Google Scholar]
doi.org/10.1021/acsami.6b00891. [PubMed] [Google Scholar]
103. Liu Y, Meng H, Konst S, et al. Injectable dopaminemodified poly (ethylene glycol) nanocomposite hydrogel with enhanced adhesive property and bioactivity. ACS Appl Mater Interfaces. 2014;6(19):16982–16992. http://dx.doi.org/10.1021/am504566v. [PMC free article] [PubMed] [Google Scholar]
104. Demirtaş T T, Irmak G, Gümüşderelioğlu M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication. 2017;9(3):035003. http://dx.doi.org/10.1088/1758-5090/aa7b1d. [PubMed] [Google Scholar]
105. Diogo G, Gaspar V, Serra I, et al. Manufacture of β-TCP/alginate scaffolds through a Fab@home model for application in bone tissue engineering. Biofabrication. 2014;6(2):025001. http://dx.doi.org/10.1088/1758-5082/6/2/025001. [PubMed] [Google Scholar]
106. Kang M-H, Jang T-S, Jung H-D, et al. Poly (ether imide)-silica hybrid coatings for tunable corrosion behavior and improved biocompatibility of magnesium implants. Bioact Mater. 2016;11(3):035003. http://dx.doi.org/10.1088/1748-6041/11/3/035003. [PubMed] [Google Scholar]
Bioact Mater. 2016;11(3):035003. http://dx.doi.org/10.1088/1748-6041/11/3/035003. [PubMed] [Google Scholar]
107. Lee H, Kim Y, Kim S, et al. Mineralized biomimetic collagen/alginate/silica composite scaffolds fabricated by a low-temperature bio-plotting process for hard tissue regeneration:fabrication, characterisation and in vitro cellular activities. J Mater Chem B Mater Biol Med. 2014;2(35):5785–5798. http://dx.doi.org/10.1039/C4TB00931B. [PubMed] [Google Scholar]
108. Wang X, Tolba E, Schröder H C, et al. Effect of bioglass on growth and biomineralization of SaOS-2 cells in hydrogel after 3D cell bioprinting. PLoS One. 2014;9(11):e112497. http://dx.doi.org/10.1371/journal.pone.0112497. [PMC free article] [PubMed] [Google Scholar]
109. Huey D J, Hu J C, Athanasiou K A. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917–921. http://dx.doi.org/10.1126/science.1222454. [PMC free article] [PubMed] [Google Scholar]
110. Bartnikowski M, Akkineni A R, Gelinsky M, et al. A hydrogel model incorporating 3D-plotted hydroxyapatite for osteochondral tissue engineering. Materials. 2016;9(4):285. http://dx.doi.org/10.3390/ma9040285. [PMC free article] [PubMed] [Google Scholar]
A hydrogel model incorporating 3D-plotted hydroxyapatite for osteochondral tissue engineering. Materials. 2016;9(4):285. http://dx.doi.org/10.3390/ma9040285. [PMC free article] [PubMed] [Google Scholar]
111. Kundu J, Shim J H, Jang J, et al. An additive manufacturingbased PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J Tissue Eng Regen Med. 2015;9(11):1286–1297. http://dx.doi.org/10.1002/term.1682. [PubMed] [Google Scholar]
112. Xu T, Binder K W, Albanna M Z, et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2012;5(1):015001. http://dx.doi.org/10.1088/1758-5082/5/1/015001. [PubMed] [Google Scholar]
113. Wei J, Wang J, Su S, et al. 3D printing of an extremely tough hydrogel. RSC Adv. 2015;5(99):81324–81329. http://dx.doi.org/10.1039/C5RA16362E. [Google Scholar]
114. Sugihara H, Toda S, Miyabara S, et al. Reconstruction of the skin in three-dimensional collagen gel matrix culture. In Vitro Cell Dev Biol Anim. 1991;27(2):142–146. http://dx.doi.org/10.1007/BF02631000. [PubMed] [Google Scholar]
In Vitro Cell Dev Biol Anim. 1991;27(2):142–146. http://dx.doi.org/10.1007/BF02631000. [PubMed] [Google Scholar]
115. Dorsett-Martin W A. Rat models of skin wound healing:A review. Wound Repair Regen. 2004;12(6):591–599. http://dx.doi.org/10.1111/j.1067-1927.2004.12601.x. [PubMed] [Google Scholar]
116. Skardal A, Mack D, Kapetanovic E, et al. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells TranslMed. 2012;1(11):792–802. http://dx.doi.org/10.5966/sctm.2012-0088. [PMC free article] [PubMed] [Google Scholar]
117. Sayyar S, Murray E, Thompson B, et al. Processable conducting graphene/chitosan hydrogels for tissue engineering. J Mater Chem B Mater Biol Med. 2015;3(3):481490. http://dx.doi.org/10.1039/C4TB01636J. [PubMed] [Google Scholar]
118. Suh J-K, Matthew H W. Application of chitosanbased polysaccharide biomaterials in cartilage tissue engineering:A review. Biomaterials. 2000;21(24):2589–2598. http://dx.doi.org/10. 1016/S0142-9612(00)00126-5. [PubMed] [Google Scholar]
1016/S0142-9612(00)00126-5. [PubMed] [Google Scholar]
119. Knowlton S, Yenilmez B, Anand S, et al. Photocrosslinkingbased bioprinting:Examining crosslinking schemes. Bioprinting. 2017;5:10–18. https:// dx.doi.org/10.1016/j.bprint.2017.03.001. [Google Scholar]
120. Nair K, Gandhi M, Khalil S, et al. Characterization of cell viability during bioprinting processes. Biotechnol J. 2009;4(8):1168–1177. http://dx.doi.org/10.1002/biot.200900004. [PubMed] [Google Scholar]
121. Arslan-Yildiz A, El Assal R, Chen P, et al. Towards artificial tissue models:Past, present, and future of 3D bioprinting. Biofabrication. 2016;8(1):014103. http://dx.doi.org/10.1088/1758-5090/8/1/014103. [PubMed] [Google Scholar]
122. Pereira R F, Bartolo P J. 3D bioprinting of photocrosslinkable hydrogel constructs. J Appl Polym Sci. 2015;132(48):42458. http://dx.doi.org/10.1002/App.42458. [Google Scholar]
123. Kirchmajer D M, Gorkin R, Panhuis M I H. An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing. J Mater Chem B Mater Biol Med. 2015;3(20):4105–4117. http://dx.doi.org/10.1039/c5tb00393h. [PubMed] [Google Scholar]
J Mater Chem B Mater Biol Med. 2015;3(20):4105–4117. http://dx.doi.org/10.1039/c5tb00393h. [PubMed] [Google Scholar]
124. Chirag Khatiwala R L, Benjamin Shepherd, Scott Dorfman, et al. 3D cell bioprinting for regenerative medicine research and therapies. Gene Ther Regul. 2012;7(1):1230004. http://dx.doi.org/10.1142/S156855↣000301. [Google Scholar]
125. Wang Z J, Jin X, Dai R, et al. An ultrafast hydrogel photocrosslinking method for direct laser bioprinting. RSC Adv. 2016;6(25):21099–21104. http://dx.doi.org/10.1039/c5ra24910d. [Google Scholar]
126. Armstrong J P K, Burke M, Carter B M, et al. 3D bioprinting using a templated porous bioink. Adv Healthc Mater. 2016;5(14):1724–1730. http://dx.doi.org/10.1002/adhm.201600022. [PubMed] [Google Scholar]
127. Cui X F, Breitenkamp K, Finn M G, et al. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A. 2012;18(11 -12):1304–1312. http://dx.doi.org/10.1089/ten.tea.2011.0543. [PMC free article] [PubMed] [Google Scholar]
128. Fedorovich N E, Oudshoorn M H, van Geemen D, et al. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials. 2009;30(3):344–353. http://dx.doi.org/10.1016/j.biomaterials.2008.09.037. [PubMed] [Google Scholar]
Fedorovich N E, Oudshoorn M H, van Geemen D, et al. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials. 2009;30(3):344–353. http://dx.doi.org/10.1016/j.biomaterials.2008.09.037. [PubMed] [Google Scholar]
129. Folkman J, Hochberg M. Self-regulation of growth in three dimensions. J Exp Med. 1973;138(4):745–753. [PMC free article] [PubMed] [Google Scholar]
130. Li S, Xiong Z, Wang X, et al. Direct fabrication of a hybrid cell/hydrogel construct by a double-nozzle assembling technology. J Bioact Compat Polym. 2009;24(3):249265. http://dx.doi.org/10.1016/j.biomaterials.2016.07.038. [Google Scholar]
131. Jia W, Gungor-Ozkerim P S, Zhang Y S, et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials. 2016;106:58–68. http://dx.doi.org/10.1016/j.biomaterials.2016.07.038. [PMC free article] [PubMed] [Google Scholar]
132. Skardal A, Zhang J, Prestwich G D. Bioprinting vessellike constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials. 2010;31(24):6173–6181. http://dx.doi.org/10.1016/j.biomaterials.2010.04.045. [PubMed] [Google Scholar]
Biomaterials. 2010;31(24):6173–6181. http://dx.doi.org/10.1016/j.biomaterials.2010.04.045. [PubMed] [Google Scholar]
133. Dolati F, Yu Y, Zhang Y, et al. In vitro evaluation of carbon-nanotube-reinforced bioprintable vascular conduits. Nanotechnology. 2014;25(14):145101. http://dx.doi.org/10.1088/0957-4484/25/14/145101. [PMC free article] [PubMed] [Google Scholar]
134. Gao B, Yang Q Z, Zhao X, et al. 4D bioprinting for biomedical applications. Trends Biotechnol. 2016;34(9):746–756. http://dx.doi.org/10.10164.tibtech.2016.03.004. [PubMed] [Google Scholar]
135. Weiss R A, Izzo E, Mandelbaum S. New design of shape memory polymers:Mixtures of an elastomeric ionomer and low molar mass fatty acids and their salts. Macromolecules. 2008;41(9):2978–2980. http://dx.doi.org/10.1021/ma8001774. [Google Scholar]
136. Leist S K, Zhou J. Current status of 4D printing technology and the potential of light-reactive smart materials as 4D printable materials. Virtual Phys Prototyp. 2016;11(4):249262. http://dx.doi.org/10.1080/17452759.2016.119↶. [Google Scholar]
2016;11(4):249262. http://dx.doi.org/10.1080/17452759.2016.119↶. [Google Scholar]
137. He H Y, Guan J J, Lee J L. An oral delivery device based on self-folding hydrogels. J Control Release. 2006;110(2):339–346. http://dx.doi.org/10.1016/jjconrel.2005.10.017. [PubMed] [Google Scholar]
138. Khoo Z X, Teoh J E M, Liu Y, et al. 3D printing of smart materials:A review on recent progresses in 4D printing. Virtual Phys Prototyp. 2015;10(3):103–122. http://dx.doi.org/10.1080/17452759.2015.1097054. [Google Scholar]
139. He Y, Wu Y, Fu J Z, et al. Developments of 3D printing microfluidics and applications in chemistry and biology:A review. Electroanalysis. 2016;28(8):1658–1678. http://dx.doi.org/10.1002/elan.201600043. [Google Scholar]
140. Lee V K, Lanzi A M, Ngo H, et al. Generation of multi-scale vascular network system within 3D hydrogel using 3D bio-printing technology. Cell Mol Bioeng. 2014;7(3):460–472. http://dx.doi.org/10.1007/s12195-014-0340-0. [PMC free article] [PubMed] [Google Scholar]
3D printed hydrogel walks in water
Researchers in the US and South Korea have learned how to 3D print complex hydrogel actuators. Based on them, the authors created several devices that are controlled by an external electric field, can grab and move objects, and also walk, according to ACS Applied Materials & Interfaces magazine .
Based on them, the authors created several devices that are controlled by an external electric field, can grab and move objects, and also walk, according to ACS Applied Materials & Interfaces magazine .
Since it is undesirable to use rigid elements in contact with the body or internal organs in medical devices, engineers develop soft devices. As a source of movement, soft robots use motors, as well as pneumatic or hydraulic actuators, which take up a lot of space and are not very reliable due to their complexity. As an alternative, the researchers suggest using artificial muscles that can contract, expand, or flex and are not made of massive structures. nine0005
A team of researchers led by Howon Lee at Rutgers University have developed a method for 3D printing complex shape hydrogel actuators. As a material, they chose an electroactivated hydrogel, which significantly changes its shape under the influence of an electric field. After entering the electrolyte solution, its carboxyl groups are ionized and many free cations appear inside the material, and the material itself becomes negatively charged. Since the concentrations of cations in the hydrogel and the surrounding electrolyte differ, osmotic pressure arises, which is compensated by water molecules from the electrolyte solution. After an electric field appears around the hydrogel, the cations are attracted to the cathode and the osmotic pressure on different sides of the hydrogel becomes different, as a result of which one of the sides begins to absorb more water and the whole structure bends. nine0005
Since the concentrations of cations in the hydrogel and the surrounding electrolyte differ, osmotic pressure arises, which is compensated by water molecules from the electrolyte solution. After an electric field appears around the hydrogel, the cations are attracted to the cathode and the osmotic pressure on different sides of the hydrogel becomes different, as a result of which one of the sides begins to absorb more water and the whole structure bends. nine0005
In order to give the hydrogel a complex shape and turn it into a functional actuator, the researchers used projection microstereolithography. During such 3D printing, the polymer precursor is in a tray with a moving substrate. An ultraviolet emitter shines on a dynamic pattern that displays the scheme of the printed layer, and reflects radiation onto the precursor. Thus, the light only hits the desired areas, which harden.
In this way, the researchers created several actuators, including a gripper for small objects, a device capable of gripping and moving objects, and a small human model that can walk in different directions.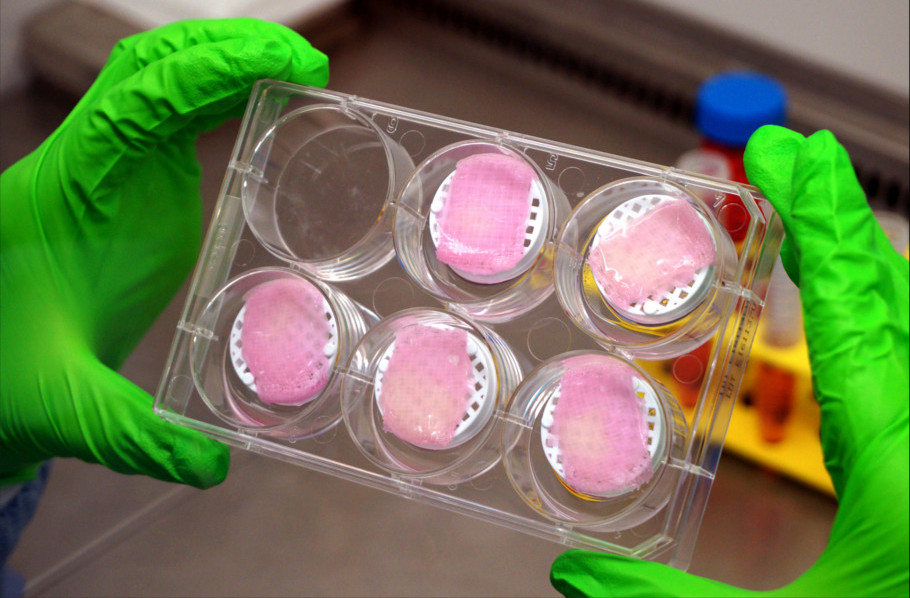 All of them work in a sodium phosphate buffer and are driven by the field generated by the electrodes. nine0005
All of them work in a sodium phosphate buffer and are driven by the field generated by the electrodes. nine0005
Last year, American engineers created soft and transparent hydraulic actuators from hydrogel. And in 2016, researchers from Harvard created a moving octopus robot, in all elements of which only soft materials are used.
Grigory Kopiev
Found a typo? Select the fragment and press Ctrl+Enter.
hybrid hydrogel and extrusion 3D printing / Sudo Null IT News71 years. For a long time, 3D printers were used exclusively for the production of functional or aesthetic prototypes, and the technology itself was called "rapid prototyping". The rapid development of computing technology has led to the emergence of various methods for implementing additive technologies: from laser stereolithography (SLA) to the more famous 3D printing (3DP).
 Another term, which appeared as early as 1894, is hydrogel - a polymer capable of absorbing water (if very exaggerated). Hydrogels, like additive technologies, have many applications: medicine, pharmacology, and even energy. So scientists from the University of North Carolina decided to combine 3D printing and hydrogel to create hydrogel structures with the desired properties. There was news about this development on Habré, but we will try to dig deeper. What does the hydrogel under study consist of, what properties can it be endowed with, and what can be made of it? We will find answers to these questions in the report of scientists. Go. nine0005
Another term, which appeared as early as 1894, is hydrogel - a polymer capable of absorbing water (if very exaggerated). Hydrogels, like additive technologies, have many applications: medicine, pharmacology, and even energy. So scientists from the University of North Carolina decided to combine 3D printing and hydrogel to create hydrogel structures with the desired properties. There was news about this development on Habré, but we will try to dig deeper. What does the hydrogel under study consist of, what properties can it be endowed with, and what can be made of it? We will find answers to these questions in the report of scientists. Go. nine0005 Study basis
To begin with, it is worth briefly explaining what a hydrogel is. This is a network of intersecting polymer chains capable of equilibrium and reversible swelling in water and aqueous solutions. The basis of the hydrogel are hydrophilic molecules.
The problem with classic hydrogels made from polymer networks in water is that they are soft and brittle; they lack elasticity and strength. Because of this, the use of hydrogels in various industries (robotics, tissue engineering, etc.) is severely limited. nine0005
Because of this, the use of hydrogels in various industries (robotics, tissue engineering, etc.) is severely limited. nine0005
The elasticity of hydrogel materials can be improved by combining interpenetrating covalent and ionic polymer networks to form highly extensible and strong structures. Another method for improving the mechanical properties of hydrogels is based on the use of fillers with a high aspect ratio (high ratio of the length of the filler to the diameter of its cross section). This allows the gel matrix to be mechanically strengthened. However, the use of a filler material other than the matrix material results in stresses on the interface surfaces that cause cracking when deformed or heated. nine0005
The opposite method is based on the use of single-polymer composites or so-called homocomposites. The mesoscale amplifying network of homocomposites is made from a material that is chemically identical to the primary matrix material. Networks of homocomposite reinforcement make it possible to modulate the mechanical properties of the primary (main) matrix without stress, delamination points, etc. It sounds very promising, but there is also a problem - the manufacture of HHG (from homocomposite hydrogel , i.e. homocomposite hydrogel) is an extremely difficult process due to the lack of methods for creating reinforcing networks with the same chemical composition as the hydrogel matrix. nine0005
It sounds very promising, but there is also a problem - the manufacture of HHG (from homocomposite hydrogel , i.e. homocomposite hydrogel) is an extremely difficult process due to the lack of methods for creating reinforcing networks with the same chemical composition as the hydrogel matrix. nine0005
In the work we are considering today, scientists describe a new type of HHG, in which both the primary gel matrix and the reinforcing network consist of sodium alginate (SA from sodium alginate ; C 6 H 9 NaO 7 ). These HHGs are reinforced with a fibrillar network of alginate soft dendritic colloids (SDC from soft dendritic colloid ). SDC is a hierarchically structured class of soft matter synthesized through a shear-induced deposition process in a turbulent medium. nine0005
The high degree of branching around the cores of SDC particles makes them morphologically similar to polymeric molecular dendrimers. However, SDCs are much larger than these dendrimers. Scientists believe that hierarchically branched SDCs could be an excellent option for effective reinforcement of composite materials. Significantly, the SDC branches cover a larger surface area, which can increase the stability of the composite by distributing the load more evenly.
Scientists believe that hierarchically branched SDCs could be an excellent option for effective reinforcement of composite materials. Significantly, the SDC branches cover a larger surface area, which can increase the stability of the composite by distributing the load more evenly.
Study results
The first step was to prepare soft alginate dendritic colloids (i.e. SDC) to serve as reinforcing meshes. For this, turbulent settling was used. To prepare the SDC hydrogel, an alginate solution (120–190 kDa) was introduced into an aqueous solution of Ca 2+ ions, which effectively bind two -COO- side groups on the alginate backbone ( 1а ).
Image #1
The precipitation process leads to the formation of SDC with a characteristic hierarchical morphology ( 1b ) with branching of different scale and generations (secondary branches) of fibers. In fact, SDCs are made up of micron-sized fibers that fork repeatedly into ever finer fibers. The outermost layer ("crown") surrounding each SDC is made up of flexible nanofibers that can be up to 10 nm thick ( 1b ). The nanofibers in the crowns endow them with physical adhesion, which is a major factor in the ability of dendricolloids to create the structural strength of the colloidal network. The final size of conventional SDCs, including their corona, is in the range of 100–500 µm. nine0005
The outermost layer ("crown") surrounding each SDC is made up of flexible nanofibers that can be up to 10 nm thick ( 1b ). The nanofibers in the crowns endow them with physical adhesion, which is a major factor in the ability of dendricolloids to create the structural strength of the colloidal network. The final size of conventional SDCs, including their corona, is in the range of 100–500 µm. nine0005
Next, the viscoelastic properties of aqueous SDC suspensions had to be evaluated. First, the scientists tested whether alginate SDC hydrogels form a colloidal network at low volume fractions in water. In theory, in aqueous suspensions, contacting SDCs will adhere strongly due to van der Waals forces to form a percolation* network of branched fiber subcontacts ( 2a ).
Percolation* - in chemistry, the phenomenon of the flow or non-flow of liquids through porous materials. nine0080Image #2
Evaluation of the storage moduli (G') and loss (G″) of SDC suspensions in the linear viscoelastic region showed that SDCs have a strong tendency to form colloidal networks.
 Dynamic modulus* : The dynamic modulus G set can be used to represent the relationship between vibration voltage and load: G' is the accumulation module; G″ is the loss modulus. nine0005
Dynamic modulus* : The dynamic modulus G set can be used to represent the relationship between vibration voltage and load: G' is the accumulation module; G″ is the loss modulus. nine0005Yield strength was observed in aqueous suspensions of 0.25 wt% SDC, i.e. at a lower concentration than most types of conventional colloidal gels.
A suspension of 1.0 wt.% SDC showed a G' value of 200 Pa, while the reported value for 1.0 wt.% alginate microgels should be 10–100 Pa. Those. SDC slurries have more pronounced solid-like characteristics than conventional alginate particle slurries.
Continuous phase HHG consists of molecular alginate gel combined with Ca 9 ions0057 2+ . First of all, the scientists analyzed the properties of molecular hydrogels containing 1.0 wt.% bound alginate, but without SDC. The hydrogel was prepared by adding CaCO 3 nanoparticles and D-gluconic acid δ-lactone (GDL) to the SA solution.
As GDL undergoes hydrolysis and lowers pH, CaCO 3 slowly releases Ca 2+ ions. After 2 hours of equilibration, data were obtained regarding the viscoelastic properties of the hydrogel ( 2c ). At a concentration of CaCO 3 above 0.05 wt.% the hydrogel behaved like a solid. With further introduction of Ca 2+ into the hydrogel, its rigidity continued to increase. But at CaCO 3 above 0.2 wt.%, syneresis (structure aging) of the hydrogel was observed, followed by water release. As a result, it was found that to maintain the stability of the hydrogel, it should contain 1.0 wt.% SA and 0.1 wt.% CaCO 3 .
As a result, the researchers had two components on hand that required combining - SA SDC (alginate soft dendritic colloids) and SA CMH molecular matrix (alginate gel bound by Ca 9 ions0057 2+ ).
A variety of hybrid HHGs have been synthesized where the total SA concentration is kept constant at 1 wt% and the ratio of SDC to CMH is varied.
Image #3All samples obtained in this way exhibited solids characteristics ( 3a ). The characteristic stress-strain curves ( 3b ) obtained from the mechanical tensile test also demonstrate that hybrid HHGs are more stiff than SDC or CMH-only gels. On chart 3c shows tensile-strain and rheometry measurements for all specimens. Analysis of these data shows that homocomposite systems containing mixed SDC and CMH exhibit a strong synergistic effect. The complex modulus (G) and Young's modulus (E) values for the homocomposite gels showed a threefold increase with maxima at low SDC to CMH ratios.
However, this cannot be attributed solely to the increase in the concentration of Ca 2+ in the homocomposite system. So the maximum shear modulus in HHG (G = 950 Pa at 0.125 wt. % SDC / 0.875 wt.
Therefore, a strong synergistic effect leading to an increase in the mechanical strength of HHG can be directly related to the physical intertwining of molecular SA and colloidal networks of SDC ( 3d ).
The resulting structure is stable in most environments, but can be easily destroyed by placing it in solutions of strong chelating agents such as EDTA (ethylenediaminetetraacetic acid; C 10 H 16 N 2 O 8 ).
Scientists note that another important feature of the developed hybrid hydrogel is the ability to change the kinetics of its gelation depending on.
First, the dependence of gelation on time was tested for three variants of samples: SDC, CMH and composite HHG (the SA concentration was the same for all - 1 wt.%).
Picture #4Chart 4a presents the results of the analysis of a pure suspension of SDC. It can be seen that the SDC immediately exhibits a solid behavior without the addition of CaCO 3 or GDL. This is explained by the fact that the formation of this network occurs due to contact splitting and interweaving of fibrillar dendricolloids.
On the other hand, pure CMH exhibits liquid behavior at first and gradually solidifies as the bonding agent Ca 2+ is released by hydrolysis.
The CMH becomes a fully connected structure after 120 minutes ( 4b ).
It is important to note that the time-dependent evolution of HHG is directly dependent on the kinetics with which SDC and CMH (the main building blocks of HHG) are assembled in the network. HHG initially solidifies due to gelation of the mechanically rigid SDC network. A stronger hydrogel then forms as the interpenetrating CMH molecular network becomes bound by Ca 2+ ions ( 4c and 4d ).
These material properties show controlled initial yield stresses and a slow increase in hydrogel elasticity over time. Therefore, such a material can be used in 3D printing, which the scientists decided to test at the next stage of the study. nine0005
The fact that the created hydrogel is a homocomposite system allows precise control of its properties. Due to this, such a hydrogel can be used in 3D printing using extrusion, which was previously an extremely difficult task. For example, both SDC and CMH are not extrudable in their pure form, unlike hybrid HHG.

The ability to control the properties of the hydrogel allows the creation of an extrusion "ink" in which the time-independent yield strength and setting time can be adjusted to suit the individual. nine0005
Synergistic effect in mixed SA-SDC composites.
Because the 3D printer applies a pressure drop that exceeds the HHG yield strength, the extruded shape is maintained by the rapid gelation of the SDC network ( 4c , video above).
Picture #5It is also important that the developed hydrogel could be used for printing under normal conditions without additional processing or material preparation ( 5a ). It is noteworthy that the G' of a pure SDC suspension (1500 Pa) is almost four orders of magnitude greater than that of the CMH mixture before the addition of GDL (0.5 Pa; 5b ).
Despite maximum HHG gel stiffness occurring at lower SDC/CMH ratios ( 4c ), HHG with higher SDC ratios produced more filaments with improved layering (video below).
3D printing of a multilayer structure by extrusion.
for 5a and 5c show the process of 3D printing a homocomposite hydrogel by direct extrusion. HHG is extruded through a nozzle (25 G, inner diameter 0.26 mm) at 140 kPa and the gel retains its shape due to its yield strength (≈80 Pa). Additional structuring in the z-direction can be achieved by stacking successive layers that have been found to adhere well to the underlying ones. The scientists were able to additively print more than 10 layers of hydrogel in the vertical direction without reducing the extrusion speed. After curing (60 minutes), the finished printed structure could be easily removed from the substrate ( 5d ). If there is a need for a structure of large dimensions, then staged extrusion is necessary, giving additional time for the gel to solidify, it is also necessary to increase the yield strength of the material by changing the composition of HHG.
For a more detailed acquaintance with the nuances of the study, I recommend looking at the report of scientists and additional materials to it.
Epilogue
In this work, scientists have demonstrated their amazing creation - a composite hydrogel, the properties of which can be manipulated depending on the needs of the end user. The hydrogel they created is excellent for 3D printing via extrusion, something that previous hydrogels could not boast of. nine0005
Scientists say that water-based materials are not very strong, they are brittle and soft, which is quite expected. However, if alginate soft dendritic colloids and alginate gel bound by Ca 2+ ions are combined, a hydrogel with increased strength can be obtained. In other words, they have combined two different hydrogels into one with properties that are superior to those of its constituents.
Applications for the new hydrogel include medicine, the food industry, and soft robotics.
But full use is still far away, since the hydrogel needs to be improved. In particular, the scientists want to modify the hydrogel so that it can be used in 3D printing of biomedical injection materials. nine0005
Thank you for your attention, stay curious and have a great week everyone. :)
Some advertising
Thank you for staying with us. Do you like our articles? Want to see more interesting content? Support us by placing an order or recommending to your friends, cloud VPS for developers from $4.99, a unique analogue of entry-level servers, which was invented by us for you: The whole truth about VPS (KVM) E5-2697 v3 (6 Cores) 10GB DDR4 480GB SSD 1Gbps from $19or how to properly divide the server? (available with RAID1 and RAID10, up to 24 cores and up to 40GB DDR4).
Dell R730xd 2 times cheaper in the Maincubes Tier IV data center in Amsterdam? Only here 2 x Intel TetraDeca-Core Xeon 2x E5-2697v3 2.6GHz 14C 64GB DDR4 4x960GB SSD 1Gbps 100 TV from $199 in the Netherlands! Dell R420 - 2x E5-2430 2.
Learn more