Stem cell 3d printing organs
Researchers take step closer to 3D printed organs with new cell-laden bioink
0Shares
Researchers from Lund University in Sweden have developed a new 3D printable bioink that could bring the 3D printing of human organs one step closer to reality.
Made up of a combination of alginate derived from seaweed and lung tissue, the bioink enables biocompatible constructs that resemble human-sized airways to be 3D printed. Once printed, the constructs support new cell and blood vessel growth in the transplanted material.
While lung tissue was the focus of the initial study, the bioink can reportedly be adapted for any tissue or organ type.
As such, the researchers, led by Associate Professor and senior author of the study Darcy Wagner, believe their work identifies a promising new class of bioinks for 3D printing functional human tissue for transplantation.
3D bioprinting human tissue
Wagner and her team began by combining alginate derived from seaweed with an extracellular matrix taken from lung tissue to form the bioink. The bioink was then laden with stem cells found in human airways and 3D printed to form complex and mechanically stable tissue constructs that mimicked these airways.
“We started small by fabricating small tubes, because this is a feature found in both airways and in the vasculature of the lung,” Wagner said. “By using our new bioink with stem cells isolated from patient airways, we were able to bioprint small airways which had multiple layers of cells and remained open over time.”
The 3D printed constructs included perfusable tubes and branching structures that spanned the anatomical length scales of human tissue, and which didn’t require external support structures.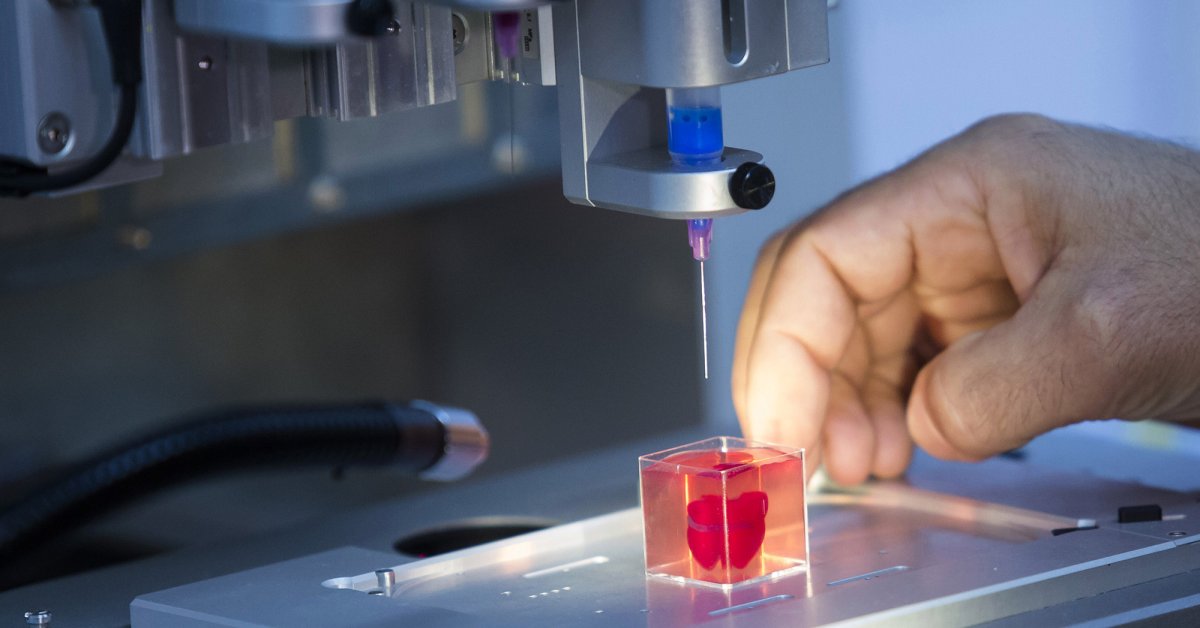 The presence of the extracellular matrix within the bioink helped to enhance the survival of human progenitor cells – descendants of stem cells that further differentiate to create specialized cell types – during the bioprinting process to enable tissue-specific cellular differentiation and vascularization of the implant within the transplant site.
The presence of the extracellular matrix within the bioink helped to enhance the survival of human progenitor cells – descendants of stem cells that further differentiate to create specialized cell types – during the bioprinting process to enable tissue-specific cellular differentiation and vascularization of the implant within the transplant site.
For the transplant site, the team used a mouse model that closely resembled the immunosuppression used in patients undergoing organ transplantation. Immunosuppression refers to the state at which your immune system is not functioning as well as it should, which can be caused by medical procedures such as this.
According to Wagner, the 3D printed constructs formed from the developed bioink suppressed the foreign body response, were pro-angiogenic and supported blood vessel formation. This is a result of the bioink’s ability to maintain its biological activity both during and after the printing process.
“These next generation bioinks also support the maturation of the airway stem cells into multiple cell types found in adult human airways, which means that less cell types need to be printed, simplifying the nozzle numbers needed to print tissue made of multiple cell types,” she explained.
High-resolution 3D printing holds the answer
In order for Wagner and her team to continue advancing and improving their newly-developed bioink, the resolution of 3D bioprinting needs to be further improved. Higher resolution printing would allow the researchers to 3D print more distal lung tissue and alveoli, which are vital for gas exchange, and to bring fully 3D printed lungs closer to reality.
“We hope that further technological improvements of available 3D printers and further bioink advances will allow for bioprinting at a higher resolution in order to engineer larger tissues which could be used for transplantation in the future,” she said. “We still have a long way to go.”
Martina De Santis, first author of the study, added: “The development of this new bioink is a significant step forward, but it is important to further validate the functionality of the small airways over time and to explore the feasibility of this approach in large animal models. ”
”
More information on the study can be found in the paper titled “Extracellular-Matric-Reinforced Bioinks for 3D Bioprinting Human Tissue”, published in the Advanced Materials journal. The study was co-authored by M. De Santis, H. Alsafadi, S. Tas, D. Bölükbas, S. Prithiviraj, I. Da Silva, M. Mittendorfer, C. Ota, J. Stegmayr, F. Daoud, M. Königshoff, K. Swärd, J. Wood, M. Tassieri, P. Bourgine, S. Lindstedt, S. Mohlin, and D. Wagner.
Human‐derived rECM hydrogels as bioinks for airways. Image via Lund University.Advances in 3D bioprinting
While cell-laden bioprinted structures hold great potential for human tissue and organ transplant, the technology is still very much in its experimental stage. The development of suitable bioinks, limited print speeds, print resolution, and restrictions on architectural complexities are all hurdles to the technology’s wider adoption.
However, there have been several significant developments in the past year that show promise in advancing bioprinting and its applications.
Scientists from the University at Buffalo have recently developed a rapid new 3D bioprinting method that could bring fully-printed human organs closer to reality. The method reportedly enables centimeter-sized hydrogels to be printed between 10-50 times faster than is possible with conventional 3D printing techniques, and also enables the production of embedded blood vessel networks.
Meanwhile, US 3D printer OEM 3D Systems announced plans to ramp up its regenerative medicine and bioprinting activities, after experiencing a breakthrough in its Print to Perfusion bioprinting platform. The system is now capable of rapidly producing full-size, vascularized lung scaffolds and will play a key role in the firm’s healthcare business going forwards.
Elsewhere, biotechnology firm United Therapeutics and Israeli bioprinting firm CollPlant expanded their partnership for the mass-manufacture of 3D printed kidneys in September 2020, although the companies have since terminated their agreement in February this year. 3D bioprinting has been deployed to arrange neural brain cells into complex patterns that could be used to model the progress of neurological diseases.
3D bioprinting has been deployed to arrange neural brain cells into complex patterns that could be used to model the progress of neurological diseases.
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows blood vessel infiltration in the 3D bioprinted constructs. Image via Lund University.
Tags 3D Systems C. Ota CollPlant D. Bölükbas Darcy Wagner F. Daoud H. Alsafadi I. Da Silva J. Stegmayr J. Wood K. Swärd Lund University M. De Santis M. Königshoff M. Mittendorfer M. Tassieri Martina De Santis P. Bourgine S. Lindstedt S. Mohlin S. Prithiviraj S. Tas United Therapeutics University at Buffalo
Hayley Everett
Hayley is a Technology Journalist for 3DPI and has a background in B2B publications spanning manufacturing, tools and cycling. Writing news and features, she holds a keen interest in emerging technologies which are impacting the world we live in.
Writing news and features, she holds a keen interest in emerging technologies which are impacting the world we live in.
3D printing | The Stem Cellar
/ Yimy Villa / Leave a comment
This image used on the cover of the American Heart Association’s Circulation Research journal is a 3D rendering of the printed heart pump developed at the University of Minnesota. The discovery could have major implications for studying heart disease.Credit: Kupfer, Lin, et al., University of Minnesota
According to the Centers for Disease Control and Prevention (CDC), heart disease is the leading cause of death for men, women, and people of most racial and ethnic groups in the United States. About 647,000 Americans die from heart disease each year, which is roughly one out of every four deaths total in the US.
In order to better study heart disease, Dr.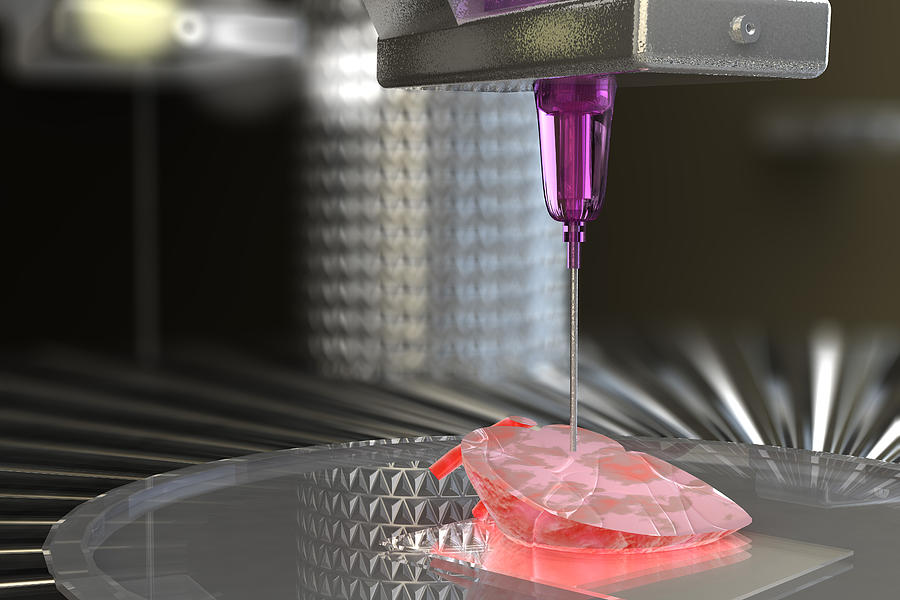 Brenda Ogle and her team at the University of Minnesota have successfully 3D printed a functioning centimeter-scale human heart pump.
Brenda Ogle and her team at the University of Minnesota have successfully 3D printed a functioning centimeter-scale human heart pump.
Previously, researchers have attempted to 3D print heart muscle cells within a 3D structure called an extracellular matrix. The heart muscle cells were made from induced pluripotent stem cells (iPSCs), a type of stem cell that can turn into virtually any kind of cell. Unfortunately, the cell density needed for the heart cells to function was never reached.
In this study. Dr. Ogle and her team made some slight changes to the process that had failed previously. First, they optimized a specialized ink made from extracellular matrix proteins. They then mixed the newly created ink with human iPSCs and used this new mixture to 3D print the chambered structure. The iPSCS were expanded to high cell densities in the structure first, and then were differentiated into heart muscle cells. The heart muscle model is about 1.5 centimeters long and was specifically designed to fit into the abdominal cavity of a mouse for future studies.
A video of this process can be seen below:
The team of researchers found that for the first time ever they could achieve the goal of high cell density to allow the cells to beat together, just like a human heart. Furthermore, this study shows how heart muscle cells can organize and work together. The iPSCs differentiating into heart muscle cells right next to each other is comparable to how stem cells grow in the body and then undergo specification to heart muscle cells.
A video of the heart pump contractions can be seen below as well:
In a press release from the University of Minnesota, Dr. Ogle elaborates on the implications of this study.
Ogle elaborates on the implications of this study.
“We now have a model to track and trace what is happening at the cell and molecular level in pump structure that begins to approximate the human heart. We can introduce disease and damage into the model and then study the effects of medicines and other therapeutics.”
The full results of this study were published in Circulation Research.
Like this:
Like Loading...
/ Yimy Villa / Leave a comment
A Sunday Afternoon on the Island of La Grande Jatte by Georges-Pierre SeuratWhen most people think of stem cells, they might conjure up an image of small dots under a microscope. It is hard to imagine these small specs being applied to three-dimensional structures. But like a pointillism painting, such as A Sunday Afternoon on the Island of La Grande Jatte by Georges-Pierre Seurat, stem cells can be used to help build things never thought possible. Two studies demonstrate this concept in very different ways.
Two studies demonstrate this concept in very different ways.
A study at MIT used nanofiber coated with muscle stem cells and mesenchymal stem cells in an effort to provide a flexible range of motion for these stem cells. Hundreds of thousands of nanofibers were twisted, resembling yarn and rope, in order to mimic the pattern found in tendons and muscle tissue throughout the body. The researchers at MIT found that the yarn like structure of the nanofibers keep the stem cells alive and growing, even as the team stretched and bent the fibers multiple times.
Normally, when a person injures these types of tissues, particularly around a major joint such as the shoulder or knee, it require surgery and weeks of limited mobility to heal properly. The MIT team hopes that their technology could be applied toward treating the site of injury while maintaining range of motion as the newly applied stem cells continue to grow to replace the injured tissue.
The MIT team hopes that their technology could be applied toward treating the site of injury while maintaining range of motion as the newly applied stem cells continue to grow to replace the injured tissue.
In an article, Dr. Ming Guo, assistant professor of mechanical engineering at MIT and one of the authors of the study, was quoted as saying,
“When you repair muscle or tendon, you really have to fix their movement for a period of time, by wearing a boot, for example. With this nanofiber yarn, the hope is, you won’t have to wearing anything like that.”
Their complete findings were published in the Proceedings of the National Academy of Sciences (PNAS).
Researchers in Germany have created transparent human organs using a new technology that could pave the way to print three-dimensional body parts such as kidneys for transplants. Above, Dr. Ali Ertuerk inspects a transparent human brain.Photo courtesy of Reuters.
In a separate study, researchers in Germany have successfully created transparent human organs, paving the way to print three-dimensional body parts. Dr. Ali Erturk at Ludwig Maximilians University in Munich, with a team of scientists, developed a technique to create a detailed blueprint of organs, including blood vessels and every single cell in its specific location. These directions were then used to print a scaffold of the organ. With the help of a 3D printer, stem cells, acting like ink in a printer, were injected into the correct positions to make the organ functional.
Dr. Ali Erturk at Ludwig Maximilians University in Munich, with a team of scientists, developed a technique to create a detailed blueprint of organs, including blood vessels and every single cell in its specific location. These directions were then used to print a scaffold of the organ. With the help of a 3D printer, stem cells, acting like ink in a printer, were injected into the correct positions to make the organ functional.
Previously, 3D-printed organs lacked detailed cellular structures because they were based on crude images from computer tomography or MRI machines. This technology has now changed that.
In an article, Dr. Erturk is quoted as saying,
“We can see where every single cell is located in transparent human organs. And then we can actually replicate exactly the same, using 3D bioprinting technology to make a real functional organ. Therefore, I believe we are much closer to a real human organ for the first time now.”
Like this:
Like Loading. ..
..
/ Pallavi Penumetcha / 4 Comments
3D printed device
Spinal cord injuries (SCIs) affect approximately 300,000 Americans, with about 18,000 new cases occurring per year. One of these patients, Jake Javier, who we have written about many times over the past several years, received ten million stem cells as part of a CIRM-funded clinical trial and a video about his first year at Cal Poly depicts how these injuries can impact someone’s life.
Currently, there is nothing that completely reverses SCI damage and most treatment is aimed at rehabilitation and empowering patients to lead as normal a life as possible under the circumstances. Improved treatment options are necessary both to improve patients’ overall quality of life, and to reduce associated healthcare costs.
Scientists at UC San Diego’s School of Medicine and Institute of Engineering in Medicine have made critical progress in providing SCI patients with hope towards a more comprehensive and longer lasting treatment option.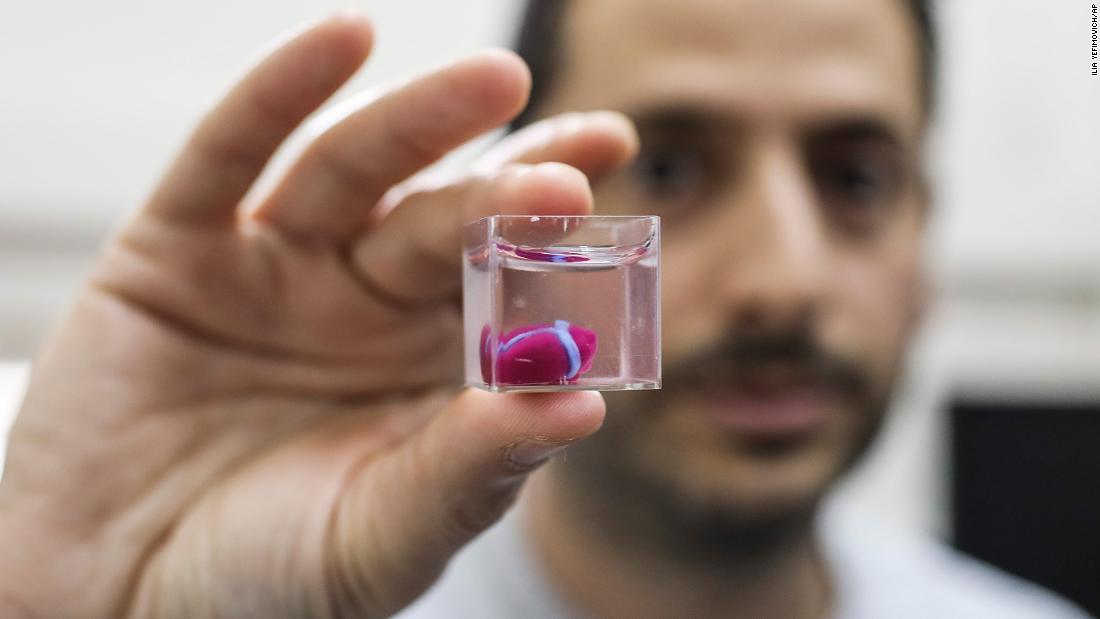
Prof. Shaochen Chen and his 3D printer
In a study partially funded by CIRM and published in Nature Medicine, Dr. Mark Tuszynski’s and Dr. Shaochen Chen’s groups used a novel 3D printing method to grow a spinal cord in the lab.
Previous studies have seen some success in lab grown neurons or nerve cells, improving SCI in animal models. This new study, however, is innovative both for the speed at which the neurons are printed, and the extent of the neuronal network that is produced.
To achieve this goal, the scientists used a biological scaffold that directs the growth of the neurons so they grow to the correct length and generate a complete neuronal network. Excitingly, their 3D printing technology was so efficient that they were able to grow implants for an animal model in 1.6 seconds, and a human-sized implant in just ten minutes, showing that their technology is scalable for injuries of different sizes.
When they tested the spinal cord implants in rats, they found that not only did the implant repair the damaged spinal cord tissue, but it also provided sustained improvement in motor function up to six months after implantation.
Just as importantly, they also observed that blood vessels had infiltrated the implanted tissue. The absence of vascularized tissue is one of the main reasons engineered implants do not last long in the host, because blood vessels are necessary to provide nutrients and support tissue growth. In this case, the animal’s body solved the problem on its own.
In a press release, one of the co-first authors of the paper, Dr. Kobi Koffler, states the importance and novelty of this work:
“This marks another key step toward conducting clinical trials to repair spinal cord injuries in people. The scaffolding provides a stable, physical structure that supports consistent engraftment and survival of neural stem cells. It seems to shield grafted stem cells from the often toxic, inflammatory environment of a spinal cord injury and helps guide axons through the lesion site completely.”
In order to make this technology viable for human clinical trials, the scientists are testing their technology in larger animal models before moving into humans, as well as investigating how to improve the longevity of the neuronal network by introducing proteins into the scaffolds.
Like this:
Like Loading...
/ Todd Dubnicoff / Leave a comment
About 120,000 people in the U.S. are on a waiting list for an organ donation and every day 22 of those people will die because there aren’t enough available organs. To overcome this organ donor crisis, bioengineers are working hard to develop 3D printing technologies that can construct tissues and organs from scratch by using cells as “bio-ink”.
Though each organ type presents its own unique set of 3D bioprinting challenges, one key hurdle they all share is ensuring that the transplanted organ is properly linked to a patient’s circulatory system, also called the vasculature. Like the intricate system of pipes required to distribute a city’s water supply to individual homes, the blood vessels of our circulatory system must branch out and reach our organs to provide oxygen and nutrients via the blood. An organ won’t last long after transplantation if it doesn’t establish this connection with the vasculature.
An organ won’t last long after transplantation if it doesn’t establish this connection with the vasculature.
Digital model of blood vessel network. Photo: Erik Jepsen/UC San Diego Publications
In a recent UC San Diego (UCSD) study, funded in part by CIRM, a team of engineers report on an important first step toward overcoming this challenge: they devised a new 3D bioprinting method to recreate the complex architecture of blood vessels found near organs. This type of 3D bioprinting approach has been attempted by other labs but these earlier methods only produced simple blood vessel shapes that were costly and took hours to fabricate. The UCSD team’s home grown 3D bioprinting process, in comparison, uses inexpensive components and only takes seconds to complete. Wei Zhu, the lead author on the Biomaterials publication, expanded on this comparison in a press release:
Wei Zhu
“We can directly print detailed microvasculature structures in extremely high resolution.
Other 3D printing technologies produce the equivalent of ‘pixelated’ structures in comparison and usually require … additional steps to create the vessels.”
As a proof of principle, the bioprinted vessel structures – made with two human cell types found in blood vessels – were transplanted under the skin of mice. After two weeks, analysis of the skin showed that the human grafts were thriving and had integrated with the mice’s blood vessels. In fact, the presence of red blood cells throughout these fused vessels provided strong evidence that blood was able to circulate through them. Despite these promising results a lot of work remains.
Microscopic 3D printed blood vessel structure. Photo: Erik Jepsen/UC San Diego Publications
As this technique comes closer to a reality, the team envisions using induced pluripotent stem cells to grow patient-specific organs and vasculature which would be less likely to be rejected by the immune system.
Shaochen Chen
“Almost all tissues and organs need blood vessels to survive and work properly.
This is a big bottleneck in making organ transplants, which are in high demand but in short supply,” says team lead Shaochen Chen. “3D bioprinting organs can help bridge this gap, and our lab has taken a big step toward that goal.”
We eagerly await the day when those transplant waitlists become a thing of the past.
Like this:
Like Loading...
/ Karen Ring / 3 Comments
As a former stem cell scientist turned science communicator, I love answering science questions no matter how complicated or bizarre. The other day my friend asked me about what CRISPR was and how scientists were using it on stem cells to help people. This got me thinking that it would be cool to do a blog on some of the latest stem cell technologies that are changing the way we do science and ultimately how we treat patients.
So in the spirit of sharing knowledge and also giving you some interesting conversation points at your next dinner party, here are five stem cell technologies that I think are pretty awesome. (As a disclaimer: this isn’t a top 5 list. I picked a few recently published studies that I thought were worth mentioning.)
(As a disclaimer: this isn’t a top 5 list. I picked a few recently published studies that I thought were worth mentioning.)
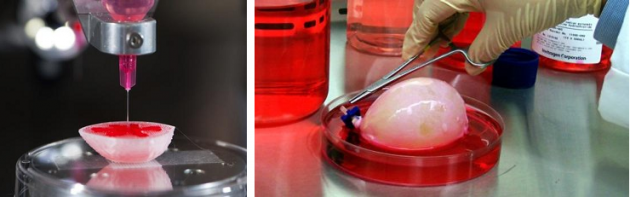
1) Need a body part? Let me print that for you.
3D printed ear. (Wake Forest University)
Scientists from Wake Forest University have developed technology to make custom-made living body parts by 3D-printing stem cells onto biodegradable scaffolds. The stem cells are printed in a hydrogel solution using a special 3D printer they call ITOP. This printer makes it possible for the printed stem cells to develop into life-sized tissues and organs that have built-in microchannels that allow blood, oxygen and other nutrients to flow through. Using the ITOP technology, the team was able to generate segments of jawbone, an ear, and muscle tissue. We wrote a blog about this fascinating technology, so check it out if you’re thirsty for more details.
2) Bio-bots controlled by light
When you think robots, you think machines and metal. But what if the robot was made out of human cells? Crazy? Not even. Scientists from the University of Illinois have made what they called “bio-bots” or tiny machines “powered by biological components.” They printed muscle cells onto flexible skeletons in the shape of rings (see GIF). The muscle cells are engineered to have light sensitive switches, so when they are exposed to light, they contract like normal muscles do. The beauty of bio-bots is that they “can sense, process, and respond to dynamic environmental signals in real time, enabling a variety of applications.” Some of these applications could include bio-bots made up of other types of tissue (brain, heart, etc.) and general use for disease research. Story credit goes to Megan Thielking’s Morning Rounds for STATnews.
But what if the robot was made out of human cells? Crazy? Not even. Scientists from the University of Illinois have made what they called “bio-bots” or tiny machines “powered by biological components.” They printed muscle cells onto flexible skeletons in the shape of rings (see GIF). The muscle cells are engineered to have light sensitive switches, so when they are exposed to light, they contract like normal muscles do. The beauty of bio-bots is that they “can sense, process, and respond to dynamic environmental signals in real time, enabling a variety of applications.” Some of these applications could include bio-bots made up of other types of tissue (brain, heart, etc.) and general use for disease research. Story credit goes to Megan Thielking’s Morning Rounds for STATnews.
Bio-bots composed of muscle cells are powered by light. (University of Illinois)
3) New way to track stem cells using MRI
Scientists from the UC San Diego School of Medicine have developed a new way to track cells in the body using magnetic resonance imaging (MRI).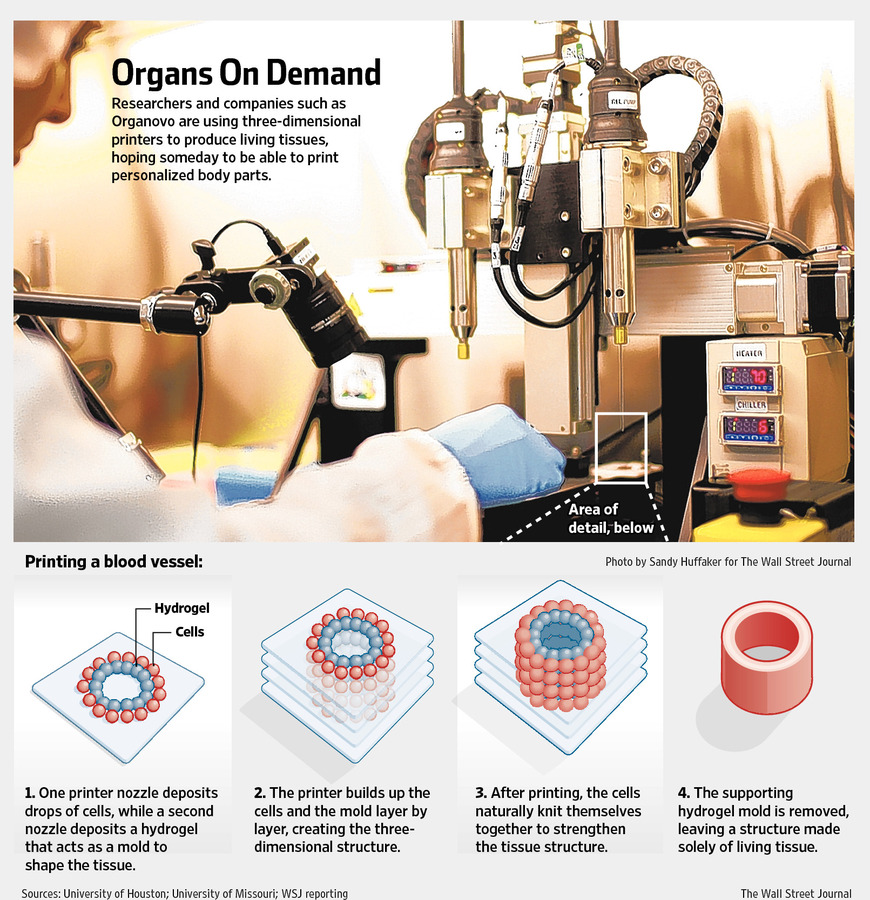 In a CIRM-funded study, the scientists made a new Fluorine-based chemical tracer that is taken in by the cells of interest. When these cells are imaged with MRI, the tracer gives off a bright and easily detectable signal. According to MNT news who covered the story, “the work is expected to enhance the progress of treatments involving stem cells and immune cells, as it will give researchers a clear picture of how cells behave after being introduced to the body.”
In a CIRM-funded study, the scientists made a new Fluorine-based chemical tracer that is taken in by the cells of interest. When these cells are imaged with MRI, the tracer gives off a bright and easily detectable signal. According to MNT news who covered the story, “the work is expected to enhance the progress of treatments involving stem cells and immune cells, as it will give researchers a clear picture of how cells behave after being introduced to the body.”
4) Engineering cells to fight cancer
Genomic modification of human stem cells by gene editing methods such as CRISPR is not a novel concept, but the technology continues to evolve at record pace and is worth mentioning. You can think of CRISPR as molecular scissors that can remove disease-causing mutations in a person’s DNA. Scientists can repair genetic mutations in human stem cells and other cell types and then use these repaired cells to replace diseased or damaged tissue or to perform therapeutic functions in patients. An article by Antonio Regalado at MIT Technology Review nicely summarizes how genetically engineered immune cells are saving the lives of cancer patients. These immune cells are engineered to recognize cancer cells (which are normally expert at evading the immune system) and when they are transplanted into cancer patients, they attack and kill off the cancer pretty effectively.
An article by Antonio Regalado at MIT Technology Review nicely summarizes how genetically engineered immune cells are saving the lives of cancer patients. These immune cells are engineered to recognize cancer cells (which are normally expert at evading the immune system) and when they are transplanted into cancer patients, they attack and kill off the cancer pretty effectively.
5) One day, stem cells will help the blind see
Artistic representation of the human eye. (Dr. Kang Zhang, Dr. Yizhi Liu)
Blindness is a big problem and stem cells are considered a promising therapeutic strategy for restoring sight in patients suffering from diseases of blindness. We covered two recent discoveries in last week’s round-up, but it never hurts to mention them again. One study from UC San Diego Health treated children suffering from cataracts. They removed the cataracts and stimulated the native stem cells in their eyes to produce new lens tissue that was able to improve their vision. The other study generated different eye parts in a dish using reprogrammed human induced pluripotent stem cells or iPS cells. They generated corneas from iPS cells and transplanted them into blind rabbits and were successful in restoring their vision. Hopefully soon stem cell technologies will advance through the clinic and provide new treatments to cure patients who’ve lost their sight.
The other study generated different eye parts in a dish using reprogrammed human induced pluripotent stem cells or iPS cells. They generated corneas from iPS cells and transplanted them into blind rabbits and were successful in restoring their vision. Hopefully soon stem cell technologies will advance through the clinic and provide new treatments to cure patients who’ve lost their sight.
Like this:
Like Loading...
/ Todd Dubnicoff / 4 Comments
“They have managed to create what appears to be the goose that really does lay golden eggs!”
That was how UK surgeon Martin Birchall described it to BBC News. The goose in this case is a 3D bioprinter, and the golden eggs are the human sized tissues that the bioprinter successfully constructed. This breakthrough for the field of tissue engineering was reported on Monday in Nature Biotechnology by a research team led by Anthony Atala, director of Wake Forest Institute for Regenerative Medicine.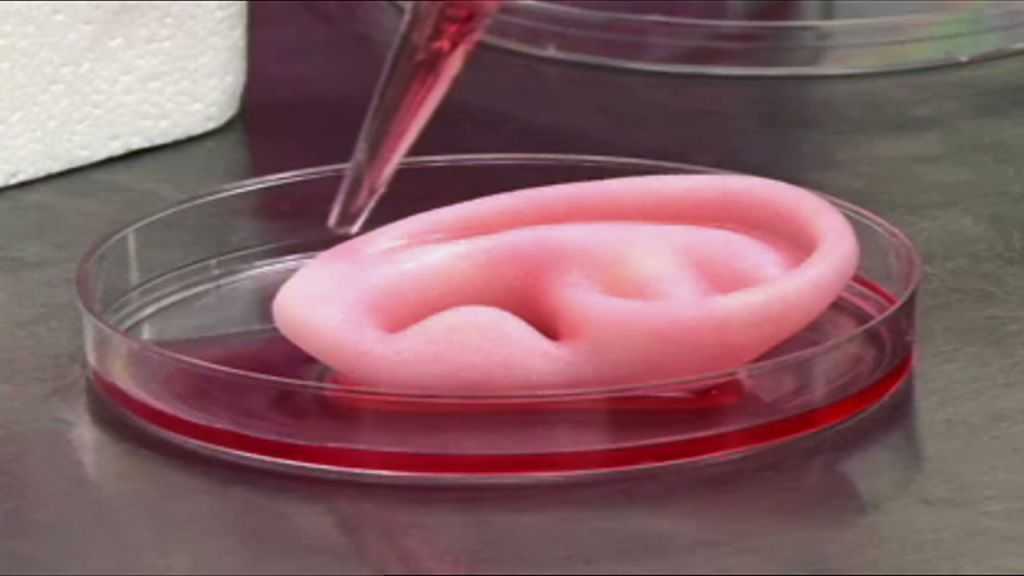
Bioprinting: yes, it’s actually a thing
The 3D bioprinting process. Image: Wake Forest, Nature Biotechnology
To some, printing human body parts may sound like a far-fetched story torn from a science fiction novel but, in reality, development of bioprinters has been underway for a number of years. The bioprinting process isn’t all that different from an inkjet printer except a mixture of gelatin, or hydrogel, and living cells is used as the “ink” to incrementally build a defined biological structure.
As amazing as this technology is, it has met some limitations. Printing cells into 2D shapes and small 3D structures is doable but a lack of structural stability limits building more complex, human-scale tissues. Also, because oxygen and nutrients can only diffuse about 0.004 inches through living tissue, the cells located inside a large bioprinted structure have trouble with long-term survival (check out yesterday’s blog for a mind-blowning story about one lab’s use of cotton candy to deal with this diffusion issue).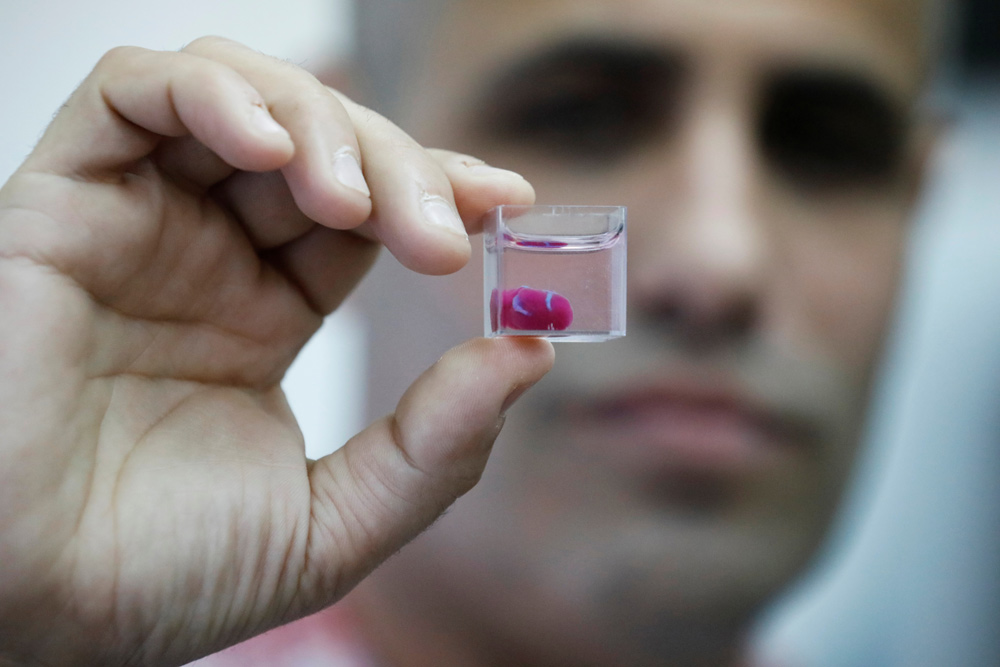 These challenges have to be overcome before 3D bioprinting can be used for the repair and replacement of human-sized tissues and organs.
These challenges have to be overcome before 3D bioprinting can be used for the repair and replacement of human-sized tissues and organs.
Enter ITOP
That’s where the Atala team’s Integrated Tissue-Organ Printer (ITOP), developed over ten years, comes into the picture. For better structural stability, the printer is configured to deliver the cell/hydrogel “bio-ink” within a stronger type of gel, with the fancy name Pluronic F127, that helps the printed cells maintain their shape during the printing process. Afterward, the Pluronic F127 scaffolding mold is simply washed away from the bioprinted tissue.
Close-up view of ITOP: a 3D bioprinter. Image: Wake Forest, Nature Biotechnology.
To ensure adequate oxygen diffusion into the bioprinted tissue, a biodegradable polymer, PCL, is dispensed from the printer at regular intervals; this creates microchannels as stand-ins for blood vessels to help oxygen and nutrients readily reach the interior areas of the tissues. As a bonus, PCL takes about 2 years to biodegrade, providing long term stability.
As a bonus, PCL takes about 2 years to biodegrade, providing long term stability.
To prove these innovations of the ITOP actually work, the team built three different tissues: a jawbone fragment, an ear, and muscle.
The making of a jawbone
For reconstruction of the jawbone, a 3D computer model was generated from actual CT scan data of a human jaw with a missing piece of bone – something that might be seen in a traumatic injury to a combat soldier (the work is funded in part by the Armed Forces Institute for Regenerative Medicine). That data was fed into the ITOP with coordinates of the precise printing pattern necessary to rebuild the shape of the jaw fragment. In this case, printing was carried out using human amniotic fluid stem cells (AFSC). With the right cues, these stem cells readily specialize into osteogenic, or bone-forming, cells.
Sure enough, 28 days after being cultured in liquid nutrients containing bone-promoting factors, the surface of the bioprinted human jaw showed calcium deposits, the tell-tale signs of bone formation. How would bioprinted bone fare in a living mammal? To find out, the researchers transplanted small discs of AFSC fabricated bone into a bone defect in mice. After 5 months, the transplanted bone was thriving with plenty of blood vessels and no necrosis, or cell death, inside the bone.
How would bioprinted bone fare in a living mammal? To find out, the researchers transplanted small discs of AFSC fabricated bone into a bone defect in mice. After 5 months, the transplanted bone was thriving with plenty of blood vessels and no necrosis, or cell death, inside the bone.
The implications of this bone study are pretty cool. In an interview with BBC news, Atala envisions a not so distant future clinical scenario:
“We’d bring the patient in, do the imaging and then we would take the imaging data and transfer it through our software to drive the printer to create a piece of jawbone that would fit precisely in the patient.”
Vincent van Gogh could have used this technology
But the possibilities don’t end with bone. Next, the team tested the ITOP’s talents at building the complex shapes of the outer human ear. In this case, the printer was loaded up with cartilage-producing cells called chondrocytes. The authors posted a fascinating video of the ear bioprinting process in their online publication.
A human-sized bioprinted ear. Image: Wake Forest, Nature Biotechnology
Image: Wake Forest, Nature Biotechnology.
After five weeks in liquid nutrients, a matrix of cartilage had grown throughout the ear. And to look at tissue growth in an animal, the ear was implanted under the skin of the mice. A couple of months after implantation, even more cartilage had formed and the shape of the ear was intact.
But wait there’s more: printing skeletal muscle
Since both the jaw bone and ear cartilage represent hard tissues, the team sought to reconstruct muscle, a soft tissue, with the ITOP. Muscle-forming cells, or myoblasts, were printed to mimic the muscle fiber bundles seen in native skeletal muscle. After growing a week in the lab under conditions that stimulate muscle cell formation, the muscle-like fibers were implanted into rats. Two week after implantation, the bioprinted muscle had not only grown into well-organized muscle fibers, they also were functional in that they were responsive to electrical stimulation.
3D bioprinting: getting closer to reality
Atala is the first to admit that a lot more testing is needed to safely bring this technology into a clinical setting for human use. But as he states in a Wake Forest press release, the ITOP brings 3D bioprinting a step closer to reality:
“This novel tissue and organ printer is an important advance in our quest to make replacement tissue for patients. It can fabricate stable, human-scale tissue of any shape. With further development, this technology could potentially be used to print living tissue and organ structures for surgical implantation.”
Like this:
Like Loading...
/ Kevin McCormack / Leave a comment
L-R Alan Tan, Sid Bommakanti, Daniel Chae – prize winning science students
A 3D printer, some old teeth, and some terrific science were enough to help three high school students develop a new way of growing bone and win a $30,000 prize in a national competition.
The three teamed up for the Siemens Competition in Math, Science & Technology, which bills itself as “the nation’s premier research competition for high school students”.
The trio includes two from the San Francisco Bay area, where we are based; Sid Bommakanti from Amador Valley High School in Pleasanton, and Alan Tan, from Irvington High School in Fremont. The third member of the team, Daniel Chae, goes to Thomas Jefferson High School for Science and Technology in Alexandria, Virginia.
The three used mesenchymal stem cells – which are capable of being turned into muscle, cartilage or bone – which they got from the dental pulp found in wisdom teeth that had been extracted.
In a story posted on the KQED website Tan says they thought it would be cool to take something that is normally thrown away, and recycle it:
“When we learned we could take stem cells from teeth—it’s actually part of medical waste—we realized could turn this into bone cells,”
The students used a 3D printer to create a kind of scaffold out of a substance called polylatctic acid – it’s an ingredient found in corn starch or sugar cane. The scaffold had a rough surface, something they hoped would help stimulate the dental pulp to grow on it and become bone.
The scaffold had a rough surface, something they hoped would help stimulate the dental pulp to grow on it and become bone.
That’s what happened. The students were able to show that their work produced small clusters of cells that were growing on the scaffold, cells that were capable of maturing into bone. This could be used to create dental implants to replace damaged teeth, and, according to Alan Tan, to repair other injuries:
“We used dental pulp stem cells so that we could regenerate bones in various parts of our body so for example we could fix bones in your jaw and tibia and other places.”
The beauty of this approach is that the scaffold and bone could be implanted in, say, the mouth and then as the scaffold disintegrates the new bone would be left in place.
While they didn’t take the top prize (a $100,000 scholarship) they did have to see off some serious competition from nearly 1,800 other student project submissions to win a Team scholarship award.
The students say they learned a lot working together, and encouraged other high school students who are interested in science to take part in competitions like this one.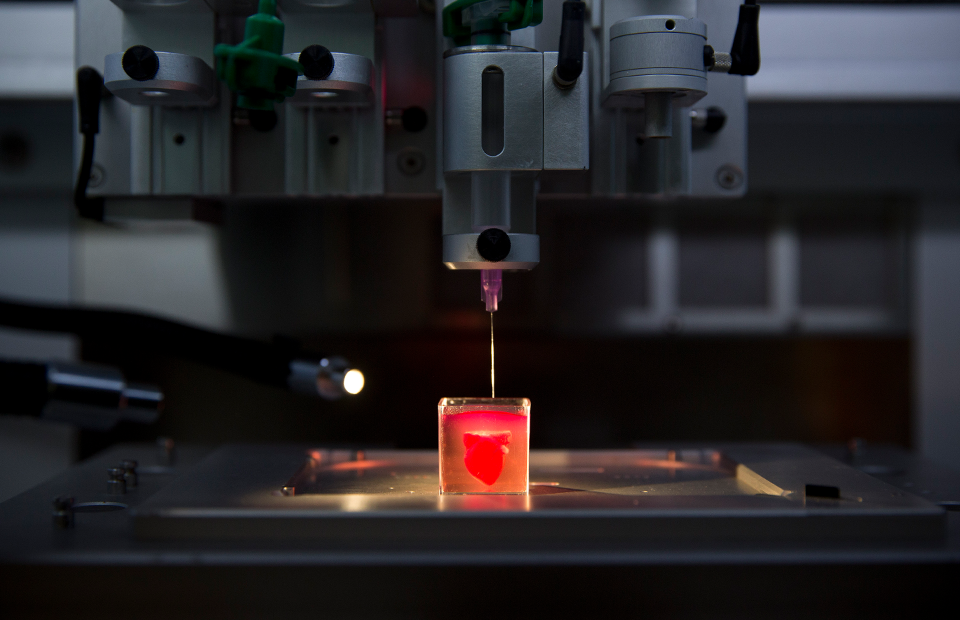
Sid Bommakanti “Both me, Alan and our other partner are interested in medicine as a whole and we wanted to make an impact on other people’s lives.”
Alan Tan: “I would say get into science early. Don’t be afraid to put yourself out there and talk to professors, talk to people, competitions like this are beneficial because they encourage students to get out there and interact with the real world.”
CIRM is helping students like these through its Stem Cell Education Portal, which includes the materials and resources that teachers need to teach high school students about stem cells. All the materials meet both state and federal guidelines.
Like this:
Like Loading...
/ Todd Dubnicoff / 2 Comments
The complex, 3D micro-anatomy of the human liver. (Image source: WikiMedia Commons)
One of the Holy Grails of stem cell research is growing body parts to replace those damaged by disease or injury. Enormous strides have been made in a key first step: mastering recipes for maturing stem cells into various specialized cell types. But a lawn of, say, liver cells in a petri dish is not a functioning liver. Organs have complex, three-dimensional structures with intricate communication between multiple cell types.
Enormous strides have been made in a key first step: mastering recipes for maturing stem cells into various specialized cell types. But a lawn of, say, liver cells in a petri dish is not a functioning liver. Organs have complex, three-dimensional structures with intricate communication between multiple cell types.
Scientists are actively devising methods to overcome this challenge. For instance, cultivating cells onto biological scaffolds help mold the cells into the shape of a particular organ or tissue. And retooled 3D printers using “bio ink” can seed layers of different cells onto these scaffolds to create specified structures.
This week, a UCSF team added an ingenious new tool to this tissue engineering tool kit. As reported on Monday in Nature Methods, the lab of Zev Gartner took advantage of DNA’s Velcro-like chemistry to build layers of different cell types in a specified pattern.
DNA – it’s not just for genetics anymore
A DNA fragment is made of two complimentary strands that bind together with high specificity.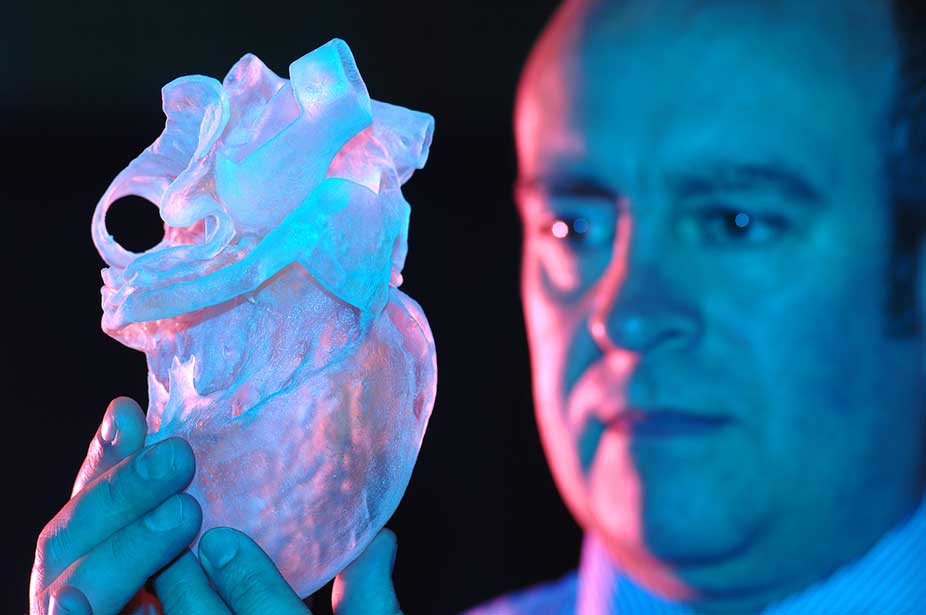 (Image source: Visionlearning)
(Image source: Visionlearning)
DNA is a molecule made of two thin strands. Each strand is specifically attracted to the other based on a unique sequence of genetic information. So if two strands of a short DNA fragment are peeled apart, they will only rejoin to each other and not some other fragment with a different sequence. While DNA usually resides in the nucleus of a cell, the team worked out a method to temporarily attach copies of a strand of DNA on the outside of, let’s call it, “cell A”. The opposite strand of that DNA fragment was attached to “cell B”. When mixed together the two cells became attached to each other via the matching DNA sequences. Other cells with different DNA fragments floated on by.
The screen shot below from a really neat time-lapse video, which accompanies the research publication, shows how a rudimentary 3D cell structure could be built with a series of different cell-DNA fragment combinations. In this case, the team first attached DNA fragments onto a petri dish in a specific pattern. At the thirty-second mark in the video, you can see that cells with matching DNA fragments have attached to the DNA on the dish.
At the thirty-second mark in the video, you can see that cells with matching DNA fragments have attached to the DNA on the dish.
This video demonstrates the assembly of 3D cell structures with the help of DNA “Velcro” (image source: Todhunter et al. Nature Methods 2015 Aug 31st)
The new technique, dubbed DNA programmed assembly of cells (DPAC), opens up a lot possibilities according to Gartner in a UCSF press release:
“We can take any cell type we want and program just where it goes. We can precisely control who’s talking to whom and who’s touching whom at the earliest stages. The cells then follow these initially programmed spatial cues to interact, move around, and develop into tissues over time.”
The Quest still continues with possible victories along the way
Of course, this advance is still a far cry from the quest for whole organs derived from stem cells. The cell assemblies using DPAC can only be grown up to about 100 microns, the thickness of a human hair. Beyond that size, the innermost cells get starved of oxygen and nutrients. Gartner says that obstacle is a current focus in the lab:
Beyond that size, the innermost cells get starved of oxygen and nutrients. Gartner says that obstacle is a current focus in the lab:
“We’re working on building functional blood vessels into these tissues. We can get the right cells in the right positions but haven’t figured out how to perfuse them with blood or a substitute efficiently yet.”
In the meantime, building these small 3D “organoids” from stem cells certainly could be put to good use as a means to test drug toxicity on human tissue or as a way to study human disease.
Related Links:
- Video: CIRM Tissue Engineering and Stem Cell Research Workshop
Like this:
Like Loading...
Search for:Enter your email address to follow The Stem Cellar Blog and receive notifications of new posts by email.
Email Address:
Like Us on FacebookMy Tweets
More Photos
CategoriesSelect Category10 Years of iPSCsAccelerating CenterAdult Stem CellsAge-Related DiseaseAgingAlliance for Regenerative MedicineAlpha Stem Cell Clinics NetworkALSAlzheimer’sAnnual Report 2017ArthritisAsk the ExpertAsterias BiotherapeuticsAutismBasic ResearchBespoke Gene Therapy ConsortiumbioengineeringBiotechnologyBlindnessBlockchainBlood stem cellsBone/Muscle/BloodBrain stem cellsBrainStorm Cell TherapeuticsBuck InstituteC. Randal MillsCaladriusCalifornia Institute for Biomedical ResearchCalifornia Institute for Regenerative MedicineCalifornia State University San MarcosCalimmuneCalTechCancerCancer Stem CellsCapricorCDICedars-Sinai Medical CenterCell TypeChildrens Hospital Los AngelesCHORICIRM 2.0CIRM Annual ReportCIRM BoardCIRM Bridges ProgramCIRM Creativity ProgramCIRM Fights CancerCIRM funded researchCIRM NewsCIRM ScholarsCIRM SPARK programCIRM Strategic PlanCity of HopeClinical Advisory Panel (CAP)Clinical TrialsCollaborative Funding PartnershipsConferencesCoriell InstituteCoronavirusCOVID-19CRISPRcystinosisDiabetesDiscovery ResearchDisease AreasDiversity, Equity, InclusionDuchenne muscular dystrophyEducationEmbryonic Stem CellsEpilepsyEthics and PolicyEventsFeaturesfetal tissueFood and Drug AdministrationForty SevenGastrointestinal DiseaseGene editingGenes + CellsGenetic DisordersGenomicsGladstone InstitutesHarvardHearing LossheartHeart Disease/Strokehematopoietic stem cellHIV/AIDSHumacyteHuntington’s DiseaseICOC Board Meetingsimmune cellsImmune DiseaseIndustry Alliance ProgramIndustry NewsInfertilityInformed consentInjuryiPS CellsiPSC RepositoryISSCR Annual MeetingjCyteKidney diseaseleukemiaLiver DiseaseLung DiseaseMacular degenerationMaria MillanMedeor TherapeuticsMental Healthmesenchymal stem cellsMitochondrial DisordersMonth of CIRMMonth of CIRMmRNAMultiple sclerosismuscle stem cellsMusclesMuscular DystrophyNational Institutes of HealthNeurological DisordersNewsObstetrics/GynecologyOrchard Therapeutics Ltd. Parkinson’sParkinson’s InstitutePatient AdvocacyPatient Advocate eventPaul KnoepflerPharmaceutical industrypluripotent stem cellsPodcastPoseida TherapeuticsPredatory stem cell clinicsProfiles in CourageRare DiseasesResearch NewsRetinitis pigmentosaRight to TrySalk InstituteSanford ConsortiumSangamo BioSciencesSCIDScientific Strategy Advisory PanelScientific Strategy Advisory PanelScripps Research InstituteSevere Combined ImmunodeficiencySickle cell diseaseSIR-Tspina bifidaSpinal cord injurySports MedicineSt. Jude’s Children’s HospitalStandards Working GroupStanford UniversityStem Cell Awareness DayStem Cell CenterStem cell championStem cell researchStem cell tourismStem cellsStem Cells in your FaceStories of HopeStrokeStudentsSuper Cells science exhibitThrowback ThursdayTranslating CenterTranslational researchUC BerkeleyUC DavisUC IrvineUC Los AngelesUC MercedUC RiversideUC San DiegoUC San FranciscoUC Santa BarbaraUC Santa CruzUncategorizedUniversity of Southern CaliforniaViaCyteVideosVirology/ImmunologyVision lossWeekly RoundupWorld Stem Cell Summit Archives Select Month October 2022 September 2022 August 2022 July 2022 June 2022 May 2022 April 2022 March 2022 February 2022 January 2022 December 2021 November 2021 October 2021 September 2021 August 2021 July 2021 June 2021 May 2021 April 2021 March 2021 February 2021 January 2021 December 2020 November 2020 October 2020 September 2020 August 2020 July 2020 June 2020 May 2020 April 2020 March 2020 February 2020 January 2020 December 2019 November 2019 October 2019 September 2019 August 2019 July 2019 June 2019 May 2019 April 2019 March 2019 February 2019 January 2019 December 2018 November 2018 October 2018 September 2018 August 2018 July 2018 June 2018 May 2018 April 2018 March 2018 February 2018 January 2018 December 2017 November 2017 October 2017 September 2017 August 2017 July 2017 June 2017 May 2017 April 2017 March 2017 February 2017 January 2017 December 2016 November 2016 October 2016 September 2016 August 2016 July 2016 June 2016 May 2016 April 2016 March 2016 February 2016 January 2016 December 2015 November 2015 October 2015 September 2015 August 2015 July 2015 June 2015 May 2015 April 2015 March 2015 February 2015 January 2015 December 2014 November 2014 October 2014 September 2014 August 2014 July 2014 June 2014 May 2014 April 2014 March 2014 February 2014 January 2014 December 2013 November 2013 October 2013 September 2013 August 2013 July 2013 June 2013 May 2013 April 2013 March 2013 February 2013 January 2013 December 2012 November 2012 October 2012 September 2012 August 2012 July 2012 June 2012 May 2012 April 2012 March 2012 February 2012 January 2012 December 2011 November 2011 October 2011 September 2011 August 2011 July 2011 June 2011 May 2011 April 2011 March 2011 February 2011 January 2011 December 2010 November 2010 October 2010 September 2010 August 2010 July 2010 June 2010 May 2010 April 2010 February 2010 January 2010 December 2009 November 2009 October 2009 September 2009 July 2009 May 2009 April 2009 March 2009 February 2009 January 2009 December 2008 November 2008 October 2008 September 2008 July 2008 June 2008 May 2008 April 2008 February 2008 September 2007 August 2007 April 2007
Parkinson’sParkinson’s InstitutePatient AdvocacyPatient Advocate eventPaul KnoepflerPharmaceutical industrypluripotent stem cellsPodcastPoseida TherapeuticsPredatory stem cell clinicsProfiles in CourageRare DiseasesResearch NewsRetinitis pigmentosaRight to TrySalk InstituteSanford ConsortiumSangamo BioSciencesSCIDScientific Strategy Advisory PanelScientific Strategy Advisory PanelScripps Research InstituteSevere Combined ImmunodeficiencySickle cell diseaseSIR-Tspina bifidaSpinal cord injurySports MedicineSt. Jude’s Children’s HospitalStandards Working GroupStanford UniversityStem Cell Awareness DayStem Cell CenterStem cell championStem cell researchStem cell tourismStem cellsStem Cells in your FaceStories of HopeStrokeStudentsSuper Cells science exhibitThrowback ThursdayTranslating CenterTranslational researchUC BerkeleyUC DavisUC IrvineUC Los AngelesUC MercedUC RiversideUC San DiegoUC San FranciscoUC Santa BarbaraUC Santa CruzUncategorizedUniversity of Southern CaliforniaViaCyteVideosVirology/ImmunologyVision lossWeekly RoundupWorld Stem Cell Summit Archives Select Month October 2022 September 2022 August 2022 July 2022 June 2022 May 2022 April 2022 March 2022 February 2022 January 2022 December 2021 November 2021 October 2021 September 2021 August 2021 July 2021 June 2021 May 2021 April 2021 March 2021 February 2021 January 2021 December 2020 November 2020 October 2020 September 2020 August 2020 July 2020 June 2020 May 2020 April 2020 March 2020 February 2020 January 2020 December 2019 November 2019 October 2019 September 2019 August 2019 July 2019 June 2019 May 2019 April 2019 March 2019 February 2019 January 2019 December 2018 November 2018 October 2018 September 2018 August 2018 July 2018 June 2018 May 2018 April 2018 March 2018 February 2018 January 2018 December 2017 November 2017 October 2017 September 2017 August 2017 July 2017 June 2017 May 2017 April 2017 March 2017 February 2017 January 2017 December 2016 November 2016 October 2016 September 2016 August 2016 July 2016 June 2016 May 2016 April 2016 March 2016 February 2016 January 2016 December 2015 November 2015 October 2015 September 2015 August 2015 July 2015 June 2015 May 2015 April 2015 March 2015 February 2015 January 2015 December 2014 November 2014 October 2014 September 2014 August 2014 July 2014 June 2014 May 2014 April 2014 March 2014 February 2014 January 2014 December 2013 November 2013 October 2013 September 2013 August 2013 July 2013 June 2013 May 2013 April 2013 March 2013 February 2013 January 2013 December 2012 November 2012 October 2012 September 2012 August 2012 July 2012 June 2012 May 2012 April 2012 March 2012 February 2012 January 2012 December 2011 November 2011 October 2011 September 2011 August 2011 July 2011 June 2011 May 2011 April 2011 March 2011 February 2011 January 2011 December 2010 November 2010 October 2010 September 2010 August 2010 July 2010 June 2010 May 2010 April 2010 February 2010 January 2010 December 2009 November 2009 October 2009 September 2009 July 2009 May 2009 April 2009 March 2009 February 2009 January 2009 December 2008 November 2008 October 2008 September 2008 July 2008 June 2008 May 2008 April 2008 February 2008 September 2007 August 2007 April 2007 - Chemistry Nobel Prize winner Carolyn Bertozzi had a hand in early stem cell research
- Smoking marijuana could be bad for your heart, but there is an unusual remedy
- Judy Chou, Ph.
 D., Appointed to Governing Board of California’s Stem Cell & Gene Therapy Agency
D., Appointed to Governing Board of California’s Stem Cell & Gene Therapy Agency
- Chemistry Nobel Prize winner Carolyn Bertozzi had a hand in early stem cell research
- Strength forged from adversity
- Judy Chou, Ph.D., Appointed to Governing Board of California’s Stem Cell & Gene Therapy Agency
Stem cells self-organized and became a material for 3D bioprinting
3D printing Biology
Complexity 4.4
Immediately after the printing process, cells condense into a densely packed line and then, through self-organization, differentiate into small intestinal epithelial cells
Matthias Lutolf et al. / Nature Materials, 2020
Scientists have developed a method for printing living tissues on a 3D bioprinter, which uses stem cells and one of their most important properties is self-organization. As reported in Nature Materials , stem cells from various tissues placed in favorable conditions self-organized and formed tissues that looked and functioned like full-fledged living tissues.
The formation of tissues in a living organism depends on intercellular contacts and the microenvironment of cells. In the process of development and life activity, cells form around themselves an extracellular matrix - a part of the tissue that serves as a mechanical support and information intermediary for cells.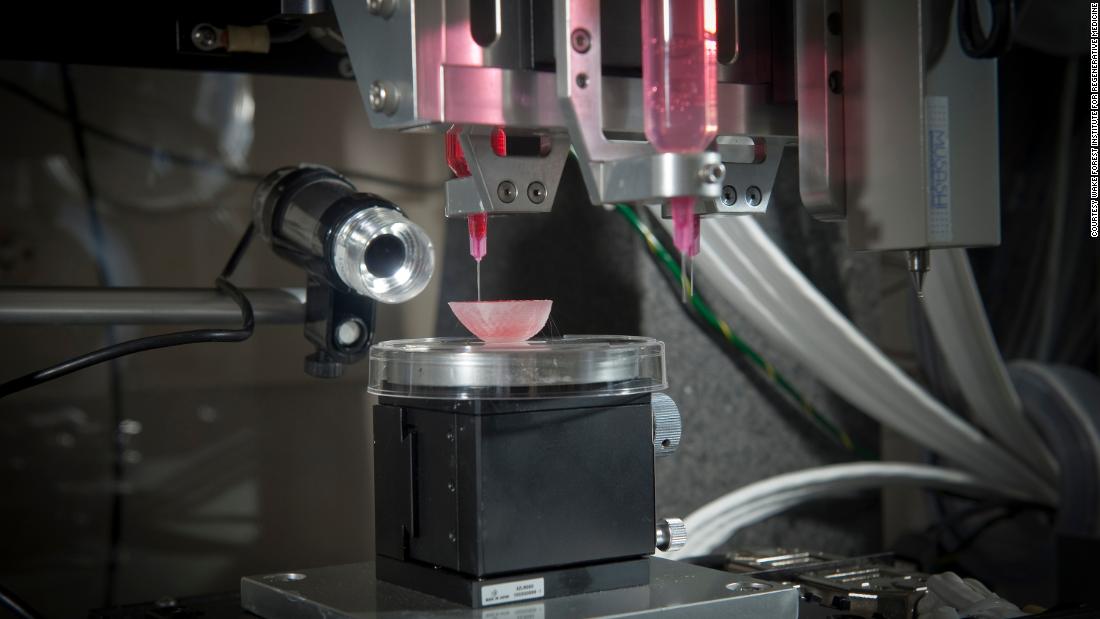 Cells are located in the matrix (respectively, in the tissue) in a spatial relationship characteristic of each organ. To be in the right place at the right time, cells express hundreds of receptors and chemicals that determine how the cell interacts with neighboring cells and the matrix. Thanks to such interactions, cells self-organize - each cell knows where it needs to be in the tissue and what it needs to do.
Cells are located in the matrix (respectively, in the tissue) in a spatial relationship characteristic of each organ. To be in the right place at the right time, cells express hundreds of receptors and chemicals that determine how the cell interacts with neighboring cells and the matrix. Thanks to such interactions, cells self-organize - each cell knows where it needs to be in the tissue and what it needs to do.
Until recently, scientists were not able to grow large organelles (more than a centimeter) using 3D bioprinting, either because the cells were too tightly attached to the environment and could not move, or the environment itself did not allow creating the necessary microenvironment. However, Matthias P. Lutolf and colleagues at the Federal Polytechnic School of Lausanne have developed a new 3D bioprinting approach that can solve these problems. The new method, which, among other advantages, allows microscopic work with cell mass and direct observation of the process of printing and growing, uses the self-organization of stem cells as the basis for growing full-fledged organs and tissues.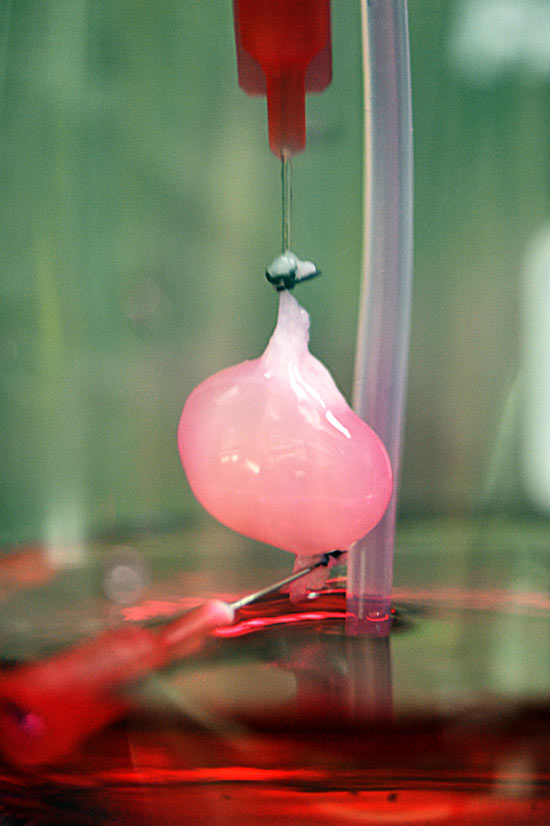 This approach allows you to repeat the natural processes of development of tissues and organs.
This approach allows you to repeat the natural processes of development of tissues and organs.
To demonstrate the potential and versatility of the new method, the scientists used human small intestine stem cells. The line-printed stem cells were placed on a nutrient medium made of hydrogel with collagen, which is similar in properties to the extracellular matrix. In this environment, cells easily moved and created a fibrous connective tissue structure around themselves, additionally turning the environment into a favorable microenvironment.
A few days later, the cells transformed into a complete and organized epithelial tube 5 to 15 millimeters long, surrounded by a specific matrix, in which scientists found the tissue organization found in the classical organelles of the small intestine. At the same time, scientists note that it was the nutrient medium and the extracellular matrix that had a great influence on the formation of the intestinal tube, which created a favorable microenvironment for cell self-organization. It is noteworthy that intercellular self-organization leveled small printing defects (for example, cell adhesion).
It is noteworthy that intercellular self-organization leveled small printing defects (for example, cell adhesion).
The scientists also managed to grow the epithelium of the mouse small intestine. At first, the stem cells were arranged in a line, but after four or six days, thanks to the self-organization of the cells, a gap appeared in this line, which turned it into a hollow tube. After another one or two days, crypts and villi, characteristic of the epithelium of the small intestine, were found in the tube, in which scientists found mature differentiated enterocytes, Paneth cells (protective cells that are found only in the small intestine), goblet and enteroendocrine cells. The entire tube responded to external stimuli — Paneth cells released bactericidal granules in response to chemical stimulation, and all cells swelled under the action of forskolin . These reactions show that the new bioprinting technique can produce engineered tissues with physiological responses reminiscent of those in living organisms.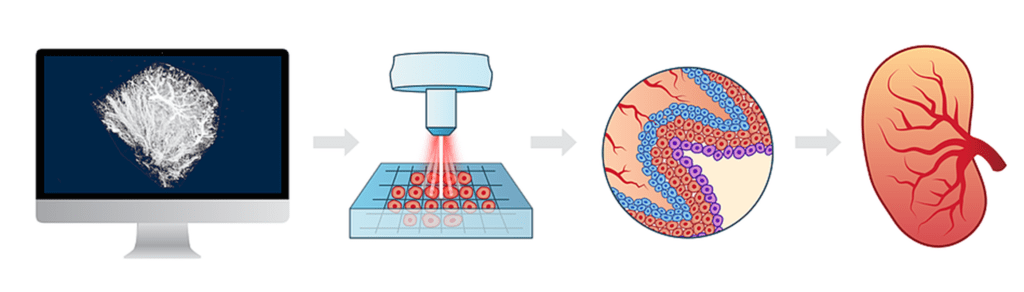
In addition, endothelial cells printed on a mixture with vascular endothelial growth factor (VEGF) formed capillary vessels de novo . Due to favorable conditions (VEGF, loose medium), the formation of capillaries was triggered at the tissue scale, which led to the formation of a vasculature with a continuous lumen.
All these experiments show that the specific local interactions that govern the self-organization of a small cell block can extend to the tissue level and form different types of tissues: both endothelial and tissues of the internal environment. Using the self-organization property of stem cells should be an important step towards growing tissues and organs in vitro , because in this case it will be possible to obtain functionally complete organs that can be used for transplantation or drug testing.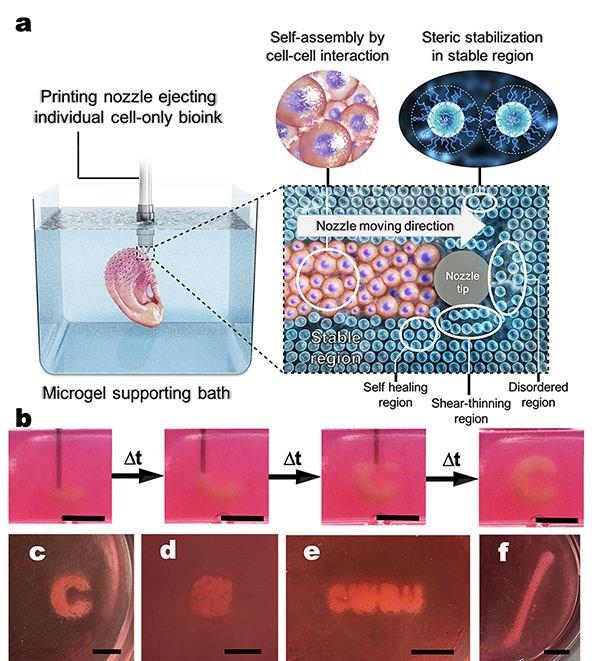
Recently, we told that another group of scientists from the same Swiss school has developed a 3D bioprinting method that differs from traditional layer-by-layer 3D printing in that the organ model is created simultaneously over the entire volume in one step, which reduces printing time to several tens of seconds.
Vyacheslav Gomenyuk
Organs created by 3D printing forced to take root
Sign up for our ”Context” newsletter: it will help you understand the events.
Image copyright Wake Forest
Photo captionAlmost any human organ can be made using 3D technology published in the journal Nature Biotechnology the results of the development of American scientists .
A breakthrough discovery makes it possible to use living tissue to repair damaged organs.
A medical professor at University College London called the new technology "the goose that lays the golden eggs.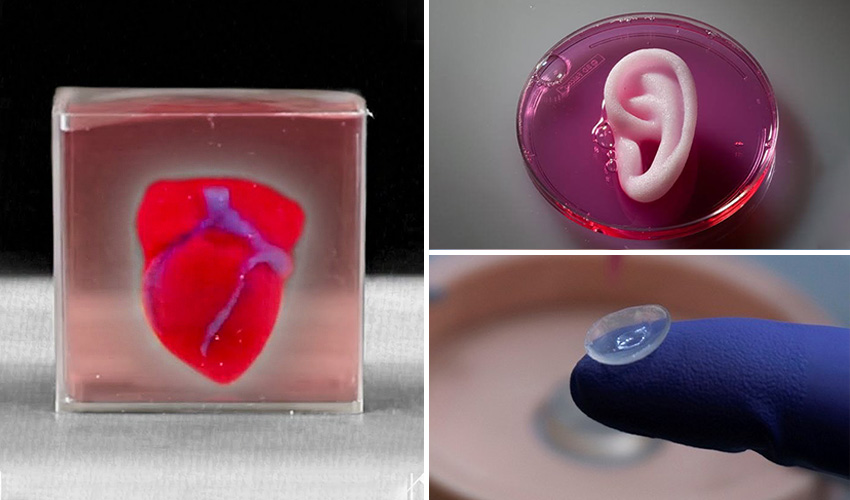 "
"
The idea of integrating individual human stem cells into a 3D printed replica of a damaged organ could revolutionize regenerative medicine.
Replacing a broken jaw, a damaged heart muscle, or restoring a missing ear to a person is easy with this technology.
Today, the main problem of transplantation of artificially regenerated organs is the difficulty of maintaining their viability - tissues over 0.2 mm thick lack oxygen and nutrients.
Sponge
A team from the American medical center Wake Forest has developed a new technique that allows the production of living tissue penetrated by microchannels using a 3D printer. The fabric has a sponge-like base, which allows nutrients and neural networks to penetrate into its structure.
Image copyright Wake Forest
Photo captionAn integrated regenerative system is used to 3D print body parts
The technology is an integrated system, part of which is responsible for tissue growth, the other is for making an exact copy of the replaced organ on a 3D printer .
The starting material consists of a biodegradable plastic that forms the outer structure of the replicated organ and a water-based gel that contains the cells and stimulates their growth.
Animal testing has shown that after implantation, the plastic is gradually degraded and replaced by a natural structural matrix of proteins produced by the cells.
Blood vessels and nerves are rotated directly into the implants.
Powerful possibilities
According to Professor Anthony Atala, lead researcher at the Wake Forest Center, it is now possible to print human tissue, but scientists want to wait until animal tests are completed to understand how durable the recreated organs are.
Image copyright Wake Forest
Image captionThis is what a broken jawbone looks like on a CT scan
Whatever the case, 3D printing opens up new possibilities for medicine. “Let’s say we have a patient with a jaw injury that is missing part of it. We do a tomography on the patient, then we send the data to the printer, and it will create the missing part of the jawbone that will completely fit the patient,” he told the BBC.
“Let’s say we have a patient with a jaw injury that is missing part of it. We do a tomography on the patient, then we send the data to the printer, and it will create the missing part of the jawbone that will completely fit the patient,” he told the BBC.
image copyrightWake Forest
Image caption,And so, the missing fragment made using a 3D printer
Technologies using biodegradable materials, which are then soaked in a solution with stem cells, are already being used.
Two years ago, Wake Forest Medical Center experimented with transplanting lab-grown female genital organs, but in general, the possibilities of such procedures are limited due to problems with maintaining cell viability.
According to Professor Atala, their recent experiment created a wide variety of tissues - muscles, soft cartilage and hard bones - which indicates the vast possibilities of the new technology.
Golden Goose
Professor Martin Birchall, a surgeon at University College London, says the results of the study are striking.