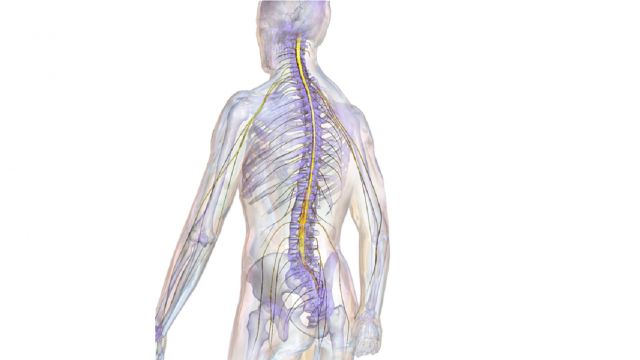
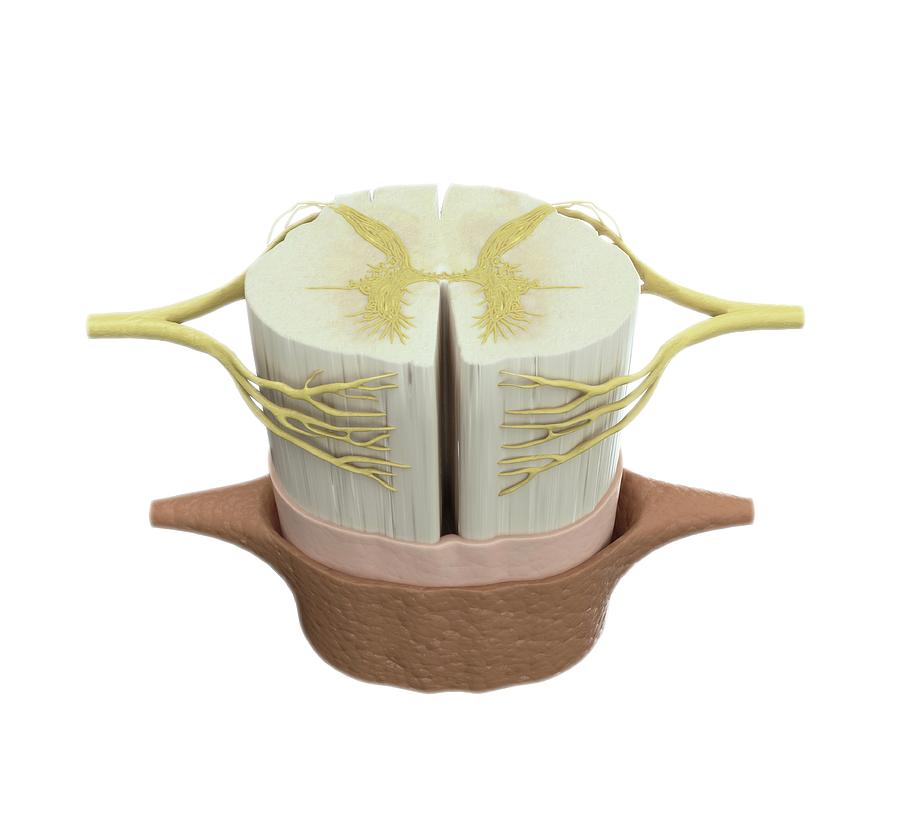
3D printed spinal cord
Matricelf eyes 3D printed human spinal cord trials after curing paralysis in mice
0Shares
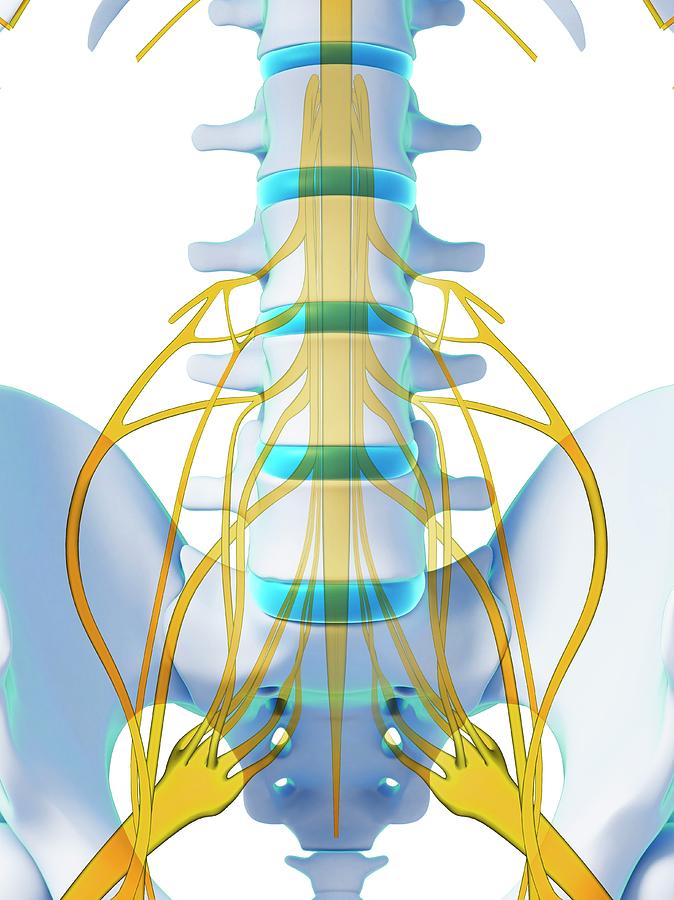
Israeli regenerative medicine firm Matricelf has successfully tested a “first-of-its-kind” 3D printed spinal cord tissue implant on paralyzed mice that has enabled them to walk again.
Leveraging 3D bioprinting technology developed at Tel Aviv University (TAU), the firm successfully 3D printed and implanted a human tissue spinal cord implant into mice with both acute and chronic paralysis. All of the mice with acute paralysis regained their ability to walk, while the success rate of those with chronic paralysis was 80 percent.
With Matricelf now gearing up to enter into human trials with its 3D printed spinal cord implants by the end of 2024, the firm says a treatment for paralysis could potentially be only a few years away.
“This is a remarkable scientific achievement that represents the potential of Matricelf’s innovative and unique technology to develop complete autologous neural implants for patients with spinal cord injury,” said Asaf Toker, Matricelf’s CEO.
The stages of Matricelf’s 3D bioprinted spinal cord process. Image via Matricelf.“The results of this study pave the way to future animal studies towards human clinical trials.”
Matricelf’s 3D bioprinting technology
Matricelf’s latest development comes hot on the heels of signing an exclusive global licensing agreement with TAU’s technology transfer firm Ramot last month for a patent-pending 3D bioprinting technology. The technology has been in development for the last decade at Professor Tal Dvir’s Department of Biotechnology at TAU, who is also one of the founders of Matricelf and the firm’s Chief Scientific Officer.
The bioprinting technique works by simultaneously 3D printing cells and extracellular matrix (ECM) derived from patients to produce living human tissues and organs. The process leverages liquid nano-particles to stabilize the printed structures and ensure high resolution and precision, which are then extracted from the structure after printing.
The technique, which is currently undergoing the patent approval process in the US and Europe, was used to produce the “world’s first” 3D printed heart in 2019, complete with cells, blood vessels, ventricles, and chambers.
The Tel Aviv University 3D bioprinted heart. Photo AFP/Jack Guez.A potential cure for paralysis
Matricelf has published the details of its groundbreaking study in a new paper, which Dvir claims marks the first time in history that engineered human tissues have generated recovery in an animal model for long-term chronic paralysis.
With this currently being the most relevant model for paralysis treatment for humans, the study holds great promise for the development of future cures leveraging 3D bioprinting technologies.
The scientists began by taking a small biopsy of belly fat tissue from a patient containing cells and ECM. After separating the cells from the ECM, the team used genetic engineering to reprogram the cells and revert them to a state resembling embryonic stem cells capable of becoming any type of cell in the body.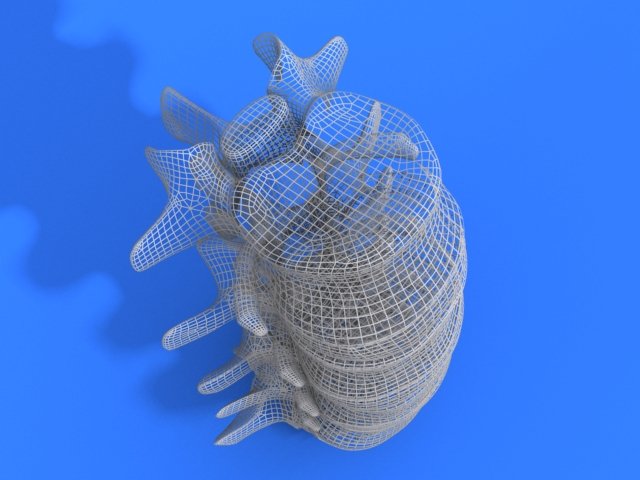
One of the main issues with donor implants is their tendency to trigger an immune response and rejection after implantation. The team hoped to overcome this by using the patient’s ECM to form the basis of a personalized hydrogel within which the stem cells were encapsulated. The cells were then turned into 3D printed implants of neuronal networks containing motor neurons through a process mimicking the embryonic development of the spinal cord.
The spinal cord implants were then implanted in a group of mice, half of which had been recently paralyzed (acute paralyzation) and half of which had been paralyzed for the equivalent of a year in human terms (chronic paralyzation).
Following implantation and a rapid rehabilitation process, all of the mice with acute paralysis regained their ability to walk, compared to 80 percent of those with chronic paralysis.
Matricelf has entered into discussions with the US Food and Drug Administration (FDA), and is now planning the first human clinical trial of its 3D printed spinal cord implants at the end of 2024.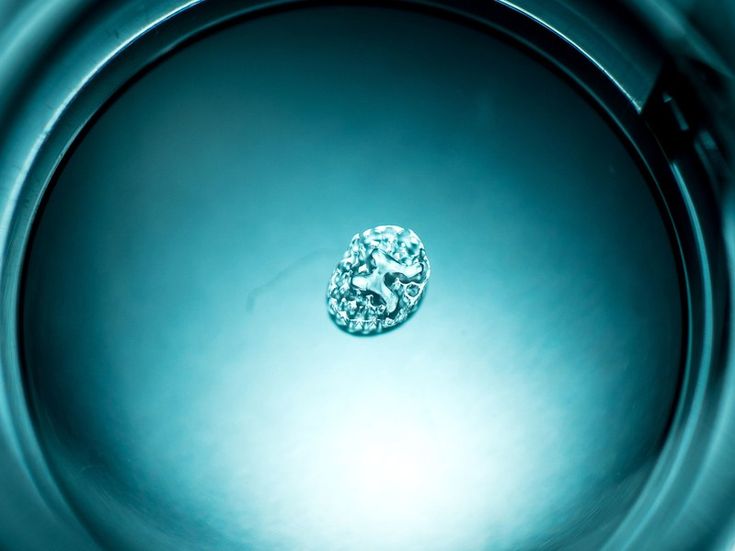 In the meantime, the firm will continue to conduct additional efficacy and safety trials on lab rats.
In the meantime, the firm will continue to conduct additional efficacy and safety trials on lab rats.
“We hope to start clinical trials in humans within the next few years, and ultimately get these patients back on their feet,” said Dvir. “The innovative technology can serve as a platform for the generation of various engineered tissues for the treatment of additional medical conditions.”
Further information on the study can be found in the paper titled: “Regenerating the injured spinal cord at the chronic phase by engineered iPSCs-derived 3D neuronal networks,” published in the Advanced Science journal. The study is co-authored by L. Wertheim, R. Edri, Y. Goldshmit, T. Kagan, N. Noor, A. Ruban, A. Shapira, I. Gat-Viks, Y. Assaf, and T. Dvir.
Generation and characterization of the spinal cord implants. Image via Matricelf.Advances in spinal cord treatments
As in many other regenerative medicine applications, 3D printing holds significant promise for improving and enhancing the success rates of spinal surgeries and treatments. While Matricelf’s breakthrough seems to be the furthest ahead in terms of realizing real-world human applications so far, there have been several other notable advances in this area in recent years.
While Matricelf’s breakthrough seems to be the furthest ahead in terms of realizing real-world human applications so far, there have been several other notable advances in this area in recent years.
Back in 2018, researchers at the University of Minnesota developed a prototype for a 3D printed scaffold with living cells that has the potential to help restore some function to patients with spinal cord injuries. Made from silicone and stem cells, the bioprinted scaffold could be placed within a patient’s spinal cord to serve as a kind of bridge between living nerve cells located around an injury. 2019, meanwhile, saw scientists from the University of California San Diego successfully 3D print a two milimeter spinal cord implant to repair spinal cord injuries in rats.
Regarding surgical applications, orthopedic implant firm 4WEB Medical added a stand-alone Anterior Spin Truss System to its 3D printed spin implant portfolio in 2020, designed to provide surgeons with greater confidence during spinal procedures.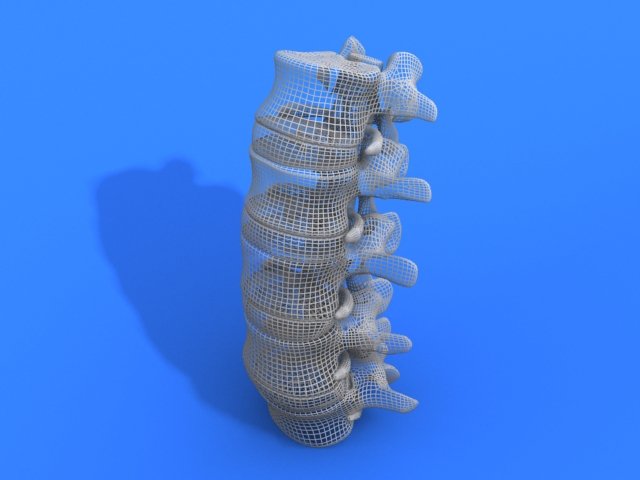
Most recently, Texas-based 3D printed orthopedic device developer Orthofix Medical launched its new FORZA Ti PLIF Spacer System designed for use in Posterior Lumbar Interbody Fusion surgeries.
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Subscribe to our YouTube channel for the latest 3D printing video shorts, reviews and webinar replays.
Featured image shows the stages of Matricelf’s 3D bioprinted spinal cord process. Image via Matricelf.
Tags 4web medical A. Ruban A. Shapira Dr Asaf Toker FDA I. Gat-Viks L. Wertheim Matricelf N. Noor Orthofix Medical Professor Tal Dvir R. Edri Ramot T. Dvir T. Kagan Tel Aviv University University of California San Diego university of minnesota Y. Assaf Y. Goldshmit
Kagan Tel Aviv University University of California San Diego university of minnesota Y. Assaf Y. Goldshmit
Hayley Everett
Hayley is a Technology Journalist for 3DPI and has a background in B2B publications spanning manufacturing, tools and cycling. Writing news and features, she holds a keen interest in emerging technologies which are impacting the world we live in.
Biomimetic 3D-printed scaffolds for spinal cord injury repair
- Letter
- Published:
- Jacob Koffler ORCID: orcid.org/0000-0002-8415-29381 na1,
- Wei Zhu ORCID: orcid.org/0000-0002-2524-08662 na1,
- Xin Qu2,
- Oleksandr Platoshyn3,
- Jennifer N.
 Dulin1,
Dulin1, - John Brock1,
- Lori Graham1,
- Paul Lu1,
- Jeff Sakamoto4,
- Martin Marsala3,
- Shaochen Chen ORCID: orcid.org/0000-0001-6876-497X2 &
- …
- Mark H. Tuszynski ORCID: orcid.org/0000-0003-4182-14811,5
Nature Medicine volume 25, pages 263–269 (2019)Cite this article
-
21k Accesses
-
299 Citations
-
270 Altmetric
-
Metrics details
Subjects
- Biomaterials
- Neuroscience
- Regenerative medicine
- Stem-cell biotechnology
- Tissue engineering
Abstract
Current methods for bioprinting functional tissue lack appropriate biofabrication techniques to build complex 3D microarchitectures essential for guiding cell growth and promoting tissue maturation1.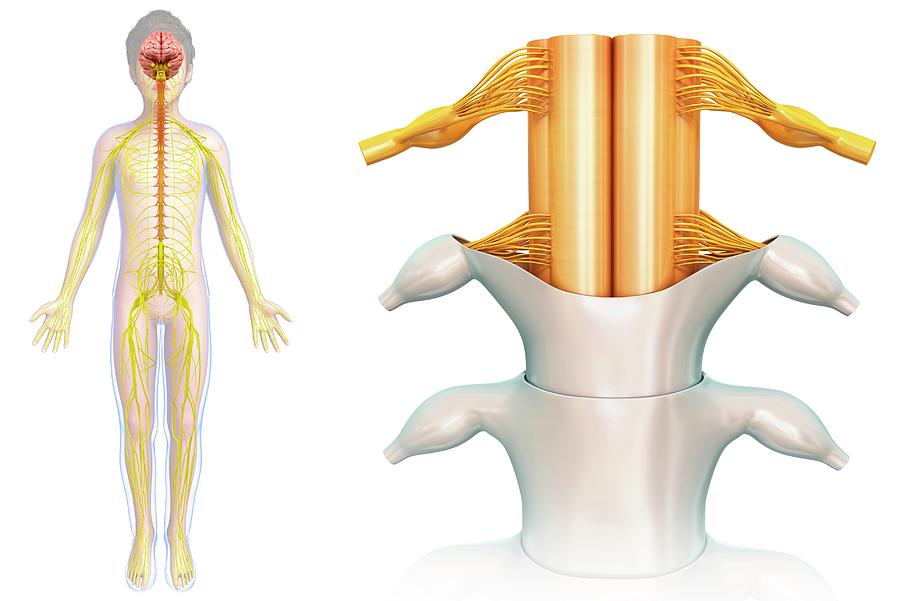 3D printing of central nervous system (CNS) structures has not been accomplished, possibly owing to the complexity of CNS architecture. Here, we report the use of a microscale continuous projection printing method (μCPP) to create a complex CNS structure for regenerative medicine applications in the spinal cord. μCPP can print 3D biomimetic hydrogel scaffolds tailored to the dimensions of the rodent spinal cord in 1.6 s and is scalable to human spinal cord sizes and lesion geometries. We tested the ability of µCPP 3D-printed scaffolds loaded with neural progenitor cells (NPCs) to support axon regeneration and form new ‘neural relays’ across sites of complete spinal cord injury in vivo in rodents1,2. We find that injured host axons regenerate into 3D biomimetic scaffolds and synapse onto NPCs implanted into the device and that implanted NPCs in turn extend axons out of the scaffold and into the host spinal cord below the injury to restore synaptic transmission and significantly improve functional outcomes.
3D printing of central nervous system (CNS) structures has not been accomplished, possibly owing to the complexity of CNS architecture. Here, we report the use of a microscale continuous projection printing method (μCPP) to create a complex CNS structure for regenerative medicine applications in the spinal cord. μCPP can print 3D biomimetic hydrogel scaffolds tailored to the dimensions of the rodent spinal cord in 1.6 s and is scalable to human spinal cord sizes and lesion geometries. We tested the ability of µCPP 3D-printed scaffolds loaded with neural progenitor cells (NPCs) to support axon regeneration and form new ‘neural relays’ across sites of complete spinal cord injury in vivo in rodents1,2. We find that injured host axons regenerate into 3D biomimetic scaffolds and synapse onto NPCs implanted into the device and that implanted NPCs in turn extend axons out of the scaffold and into the host spinal cord below the injury to restore synaptic transmission and significantly improve functional outcomes. Thus, 3D biomimetic scaffolds offer a means of enhancing CNS regeneration through precision medicine.
Thus, 3D biomimetic scaffolds offer a means of enhancing CNS regeneration through precision medicine.
This is a preview of subscription content, access via your institution
Relevant articles
Open Access articles citing this article.
-
Extracellular matrix-inspired hydrogel of hyaluronan and gelatin crosslinked via a Link module with a transglutaminase reactive sequence
- Masashi Okawa
- , Aki Tanabe
- … Taichi Ito
Communications Materials Open Access 30 October 2022
-
GelMA-MXene hydrogel nerve conduits with microgrooves for spinal cord injury repair
- Jiaying Cai
- , Hui Zhang
- … Renjie Chai
Journal of Nanobiotechnology Open Access 28 October 2022
-
Micropattern-based nerve guidance conduit with hundreds of microchannels and stem cell recruitment for nerve regeneration
- DoYeun Park
- , Donghak Kim
- … Soo Hyun Kim
npj Regenerative Medicine Open Access 20 October 2022
Access options
Subscribe to Journal
Get full journal access for 1 year
99,00 €
only 8,25 € per issue
Subscribe
Tax calculation will be finalised during checkout.
Buy article
Get time limited or full article access on ReadCube.
$32.00
Buy
All prices are NET prices.
Fig. 1: The 3D-printed scaffold mimics the spinal cord architecture.Fig. 2: Four weeks in vivo performance of empty 3D-printed scaffold implants.Fig. 3: Four weeks in vivo performance of NPC-loaded 3D-printed scaffold implants.Fig. 4: Long-term in vivo studies of 3D-printed scaffolds loaded with NPCs.Data availability
Data supporting the findings of this study are available from [email protected] on reasonable request. All requests for materials and data are promptly reviewed by the Office of Innovation and Commercialization—University of California San Diego to verify whether the request is subject to any intellectual property or confidentiality obligations. Any materials and data that can be shared will be released via a Material Transfer Agreement.
References
Kadoya, K., et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat. Med. 22, 479–487 (2016).
Lu, P. et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 150, 1264–1273 (2012).
Article CAS Google Scholar
NSCISC Annual Statistical Report - Model Systems Public Version (National Spinal Cord Injury Statistical Center, University of Alabama at Birmingham, 2014).
Murphy, S. V. & Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 32, 773–785 (2014).
Article CAS Google Scholar
Soman, P., Chung, P. H., Zhang, A. P. & Chen, S. Digital microfabrication of user-defined 3D microstructures in cell-laden hydrogels. Biotechnol. Bioeng. 110, 3038–3047 (2013).
Article CAS Google Scholar
Dalton, P. D., Flynn, L. & Shoichet, M. S. Manufacture of poly(2-hydroxyethyl methacrylate-co-methyl methacrylate) hydrogel tubes for use as nerve guidance channels. Biomaterials 23, 3843–3851 (2002).
Article CAS Google Scholar
Hung, T. K., Chang, G. L., Lin, H. S., Walter, F. R. & Bunegin, L. Stress-strain relationship of the spinal cord of anesthetized cats. J. Biomech. 14, 269–276 (1981).
Article CAS Google Scholar
Tsai, E. C., Dalton, P. D., Shoichet, M. S. & Tator, C.
 H. Synthetic hydrogel guidance channels facilitate regeneration of adult rat brainstem motor axons after complete spinal cord transection. J. Neurotrauma 21, 789–804 (2004).
H. Synthetic hydrogel guidance channels facilitate regeneration of adult rat brainstem motor axons after complete spinal cord transection. J. Neurotrauma 21, 789–804 (2004).Article Google Scholar
Koffler, J., Samara, R. F. & Rosenzweig, E. S. Using templated agarose scaffolds to promote axon regeneration through sites of spinal cord injury. Methods Mol. Biol. 1162, 157–165 (2014).
Article CAS Google Scholar
Gao, M. et al. Templated agarose scaffolds for the support of motor axon regeneration into sites of complete spinal cord transection. Biomaterials 34, 1529–1536 (2013).
Article CAS Google Scholar
Gros, T., Sakamoto, J. S., Blesch, A., Havton, L. A. & Tuszynski, M. H. Regeneration of long-tract axons through sites of spinal cord injury using templated agarose scaffolds.
 Biomaterials 31, 6719–6729 (2010).
Biomaterials 31, 6719–6729 (2010).Article CAS Google Scholar
Stokols, S. et al. Templated agarose scaffolds support linear axonal regeneration. Tiss. Eng. 12, 2777–2787 (2006).
Article CAS Google Scholar
Stokols, S. & Tuszynski, M. H. The fabrication and characterization of linearly oriented nerve guidance scaffolds for spinal cord injury. Biomaterials 25, 5839–5846 (2004).
Article CAS Google Scholar
Stokols, S. & Tuszynski, M. H. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials 27, 443–451 (2006).
Article CAS Google Scholar
Lu, P. et al. Prolonged human neural stem cell maturation supports recovery in injured rodent CNS. J. Clin. Invest. 127, 3287–3299 (2017).
Article Google Scholar
Lu, P. et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron 83, 789–796 (2014).
Article CAS Google Scholar
Li, J. & Lepski, G. Cell transplantation for spinal cord injury: a systematic review. Biomed. Res. Int. 2013, 786475 (2013).
PubMed PubMed Central Google Scholar
Park, S. S. et al. Comparison of canine umbilical cord blood-derived mesenchymal stem cell transplantation times: involvement of astrogliosis, inflammation, intracellular actin cytoskeleton pathways, and neurotrophin-3.
 Cell Transplant. 20, 1867–1880 (2011).
Cell Transplant. 20, 1867–1880 (2011).Article Google Scholar
Peron, S. et al. A delay between motor cortex lesions and neuronal transplantation enhances graft integration and improves repair and recovery. J. Neurosc. 37, 1820–1834 (2017).
Article CAS Google Scholar
Wang, L. et al. Early administration of tumor necrosis factor-alpha antagonist promotes survival of transplanted neural stem cells and axon myelination after spinal cord injury in rats. Brain Res. 1575, 87–100 (2014).
Article CAS Google Scholar
Yu, D. et al. Blockade of peroxynitrite-induced neural stem cell death in the acutely injured spinal cord by drug-releasing polymer. Stem Cells 27, 1212–1222 (2009).
Article CAS Google Scholar
Karimi-Abdolrezaee, S., Eftekharpour, E., Wang, J., Morshead, C. M. & Fehlings, M. G. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J. Neurosci. 26, 3377–3389 (2006).
Article CAS Google Scholar
Zhu, Y., Uezono, N., Yasui, T. & Nakashima, K. Neural stem cell therapy aiming at better functional recovery after spinal cord injury. Dev. Dyn. 247, 75–84 (2018).
Article Google Scholar
Fehlings, M. G., Sekhon, L. H. & Tator, C. The role and timing of decompression in acute spinal cord injury: what do we know? What should we do? Spine 26, S101–S110 (2001).
Article CAS Google Scholar
Tator, C.
 H., Fehlings, M. G., Thorpe, K. & Taylor, W. Current use and timing of spinal surgery for management of acute spinal surgery for management of acute spinal cord injury in North America: results of a retrospective multicenter study. J. Neurosurg. 91, 12–18 (1999).
H., Fehlings, M. G., Thorpe, K. & Taylor, W. Current use and timing of spinal surgery for management of acute spinal surgery for management of acute spinal cord injury in North America: results of a retrospective multicenter study. J. Neurosurg. 91, 12–18 (1999).CAS PubMed Google Scholar
Okada, S. The pathophysiological role of acute inflammation after spinal cord injury. Inflamm. Regen. 36, 20 (2016).
Article Google Scholar
Ciranna, L. Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: implications in physiological functions and in pathology. Curr. Neuropharmacol. 4, 101–114 (2006).
Article CAS Google Scholar
Harris, K. M. & Weinberg, R. J. Ultrastructure of synapses in the mammalian brain.
 Cold Spring Harb. Perspect. Biol. 4, a005587 (2012).
Cold Spring Harb. Perspect. Biol. 4, a005587 (2012).Article Google Scholar
Scannevin, R. H. & Huganir, R. L. Postsynaptic organization and regulation of excitatory synapses. Nat. Rev. Neurosci. 1, 133–141 (2000).
Article CAS Google Scholar
Armulik, A. et al. Pericytes regulate the blood–brain barrier. Nature 468, 557–561 (2010).
Article CAS Google Scholar
Weiss, N., Miller, F., Cazaubon, S. & Couraud, P. O. The blood–brain barrier in brain homeostasis and neurological diseases. Biochim. Biophys. Acta 1788, 842–857 (2009).
Article CAS Google Scholar
Basso, D. M., Beattie, M. S. & Bresnahan, J.
 C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 139, 244–256 (1996).
C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 139, 244–256 (1996).Article CAS Google Scholar
Iyer, S., Maybhate, A., Presacco, A. & All, A. H. Multi-limb acquisition of motor evoked potentials and its application in spinal cord injury. J. Neurosci. Methods 193, 210–216 (2010).
Article Google Scholar
van Gorp, S. et al. Amelioration of motor/sensory dysfunction and spasticity in a rat model of acute lumbar spinal cord injury by human neural stem cell transplantation. Stem Cell Res. Ther. 4, 57 (2013).
Article Google Scholar
Olson, H. E. et al. Neural stem cell- and Schwann cell-loaded biodegradable polymer scaffolds support axonal regeneration in the transected spinal cord.
 Tissue. Eng. A 15, 1797–1805 (2009).
Tissue. Eng. A 15, 1797–1805 (2009).Article CAS Google Scholar
Pawar, K. et al. Biomaterial bridges enable regeneration and re-entry of corticospinal tract axons into the caudal spinal cord after SCI: Association with recovery of forelimb function. Biomaterials 65, 1–12 (2015).
Article CAS Google Scholar
Wong, D. Y. et al. Macro-architectures in spinal cord scaffold implants influence regeneration. J. Neurotrauma 25, 1027–1037 (2008).
Article Google Scholar
Anderson, M. A. et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature 532, 195–200 (2016).
Article CAS Google Scholar
Filous, A. R. & Silver, J. Targeting astrocytes in CNS injury and disease: a translational research approach. Prog. Neurobiol. 144, 173–187 (2016).
Article CAS Google Scholar
Fairbanks, B. D., Schwartz, M. P., Bowman, C. N. & Anseth, K. S. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. Biomaterials 30, 6702–6707 (2009).
Article CAS Google Scholar
Yu, C. G., Joshi, A. & Geddes, J. W. Intraspinal MDL28170 microinjection improves functional and pathological outcome following spinal cord injury. J. Neurotrauma 25, 833–840 (2008).
Article CAS Google Scholar
Grill, R., Murai, K.
 , Blesch, A., Gage, F. H. & Tuszynski, M. H. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J. Neurosci. 17, 5560–5572 (1997).
, Blesch, A., Gage, F. H. & Tuszynski, M. H. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J. Neurosci. 17, 5560–5572 (1997).Article CAS Google Scholar
Download references
Acknowledgements
We thank J. Liu for materials synthesis, J. Li, D. Xue and S. You for helpful discussion and CAD design, and R. Anderson for assistance in scanning electron microscopy. This work was supported in part by the NIH (R01EB021857, R21HD090662), the NSF (1547005, 1644967), the California Institute for Regenerative Medicine (RT3–07899) and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. The electron micrographs were taken in the Cellular and Molecular Medicine Electron microscopy core facility, which is supported in part by National Institutes of Health Award number S10OD023527.
Author information
Author notes
These authors contributed equally: Jacob Koffler and Wei Zhu.
Authors and Affiliations
Department of Neuroscience, University of California San Diego, La Jolla, CA, USA
Jacob Koffler, Jennifer N. Dulin, John Brock, Lori Graham, Paul Lu & Mark H. Tuszynski
Department of NanoEngineering, University of California San Diego, La Jolla, CA, USA
Wei Zhu, Xin Qu & Shaochen Chen
Department of Anesthesiology, University of California San Diego, La Jolla, CA, USA
Oleksandr Platoshyn & Martin Marsala
Mechanical Engineering Department, University of Michigan, Ann Arbor, MI, USA
Jeff Sakamoto
Veterans Affairs Medical Center, San Diego, CA, USA
Mark H. Tuszynski
Authors
- Jacob Koffler
View author publications
You can also search for this author in PubMed Google Scholar
- Wei Zhu
View author publications
You can also search for this author in PubMed Google Scholar
- Xin Qu
View author publications
You can also search for this author in PubMed Google Scholar
- Oleksandr Platoshyn
View author publications
You can also search for this author in PubMed Google Scholar
- Jennifer N.
 Dulin
DulinView author publications
You can also search for this author in PubMed Google Scholar
- John Brock
View author publications
You can also search for this author in PubMed Google Scholar
- Lori Graham
View author publications
You can also search for this author in PubMed Google Scholar
- Paul Lu
View author publications
You can also search for this author in PubMed Google Scholar
- Jeff Sakamoto
View author publications
You can also search for this author in PubMed Google Scholar
- Martin Marsala
View author publications
You can also search for this author in PubMed Google Scholar
- Shaochen Chen
View author publications
You can also search for this author in PubMed Google Scholar
- Mark H. Tuszynski
View author publications
You can also search for this author in PubMed Google Scholar
Contributions
J. K. and W.Z. contributed equally to this work. J.K. managed the project, designed the study and scaffold, performed in vivo surgery, anatomical analyses and functional testing, and prepared the manuscript. W.Z. desgined and printed scaffolds and prepared the manuscript. X.Q. supported scaffold design and printing and reviewed the manuscript. O.P. and M.M. performed electrophysiology J.D. and J.B. traced the corticospinal system. L.G. and P.L. performed surgeries. J.S. prepared agarose scaffolds. S.C. supervised scaffold development and prepared the manuscript. M.H.T. managed the project, reviewed data and prepared the manuscript.
K. and W.Z. contributed equally to this work. J.K. managed the project, designed the study and scaffold, performed in vivo surgery, anatomical analyses and functional testing, and prepared the manuscript. W.Z. desgined and printed scaffolds and prepared the manuscript. X.Q. supported scaffold design and printing and reviewed the manuscript. O.P. and M.M. performed electrophysiology J.D. and J.B. traced the corticospinal system. L.G. and P.L. performed surgeries. J.S. prepared agarose scaffolds. S.C. supervised scaffold development and prepared the manuscript. M.H.T. managed the project, reviewed data and prepared the manuscript.
Corresponding authors
Correspondence to Jacob Koffler, Shaochen Chen or Mark H. Tuszynski.
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9
Reporting Summary
Supplementary Video 1
3D printing of biomimetic spinal cord scaffold
Rights and permissions
Reprints and Permissions
About this article
This article is cited by
-
Multimodal therapy strategies based on hydrogels for the repair of spinal cord injury
- Yan Wang
- Hong-Qian Lv
- Peng Zhang
Military Medical Research (2022)
-
GelMA-MXene hydrogel nerve conduits with microgrooves for spinal cord injury repair
- Jiaying Cai
- Hui Zhang
- Renjie Chai
Journal of Nanobiotechnology (2022)
-
Effects of biological sex mismatch on neural progenitor cell transplantation for spinal cord injury in mice
- Michael Pitonak
- Miriam Aceves
- Jennifer N.
 Dulin
Dulin
Nature Communications (2022)
-
Micropattern-based nerve guidance conduit with hundreds of microchannels and stem cell recruitment for nerve regeneration
- DoYeun Park
- Donghak Kim
- Soo Hyun Kim
npj Regenerative Medicine (2022)
-
Extracellular matrix-inspired hydrogel of hyaluronan and gelatin crosslinked via a Link module with a transglutaminase reactive sequence
- Masashi Okawa
- Aki Tanabe
- Taichi Ito
Communications Materials (2022)
3D-printed scaffold for nerve cells will help "repair" the spinal cord
TECHNOLOGIES
Reader mode enabled
Reading mode enlarges the text, removes everything superfluous from the page and makes it possible to focus on the material. Here you can turn it off at any time.
Here you can turn it off at any time.
Reading mode
Why is this necessary?
Living donor cells were placed on a 3D-printed silicone “scaffold”, which will subsequently grow and unite with neurons. This will repair damage to the spinal cord.
For the first time, scientists managed to keep almost 75% of highly vulnerable cells alive during 3D printing. Along with this, they observed how a number of cells grew into healthy neurons. After the scientists forced the cells to grow in the required direction and were able to maintain their sufficient volume for a while, until contact with the living tissues of the healed area occurs.
What's next?
Now scientists need to study how prostheses, in which artificial cells are located, take root and what connections are formed with the neurons of the body.
This enhanced image shows living cells that survived the 3D printing process. Neural stem cells derived from adult human cells were 3D printed and then differentiated into active nerve cells in the lab.
It is too early to talk about the treatment of people whose limbs do not work due to injuries. Scientists expect that they will be able to help restore the work of some muscles, the bladder and alleviate pain.
Found a mistake? Select it and press Ctrl+Enter
#Center #Enter #IT #youtube #Micro #Brain #Print #Process #Repair #Styles #University #Scientists
READ ALSO
BUSINESS
April 28, 2022, 10:00 3 min reading
BUSINESS
April 25, 2022, 14:00 7 min reading
BUSINESS
January 20, 2022, 10:15 1 min read
BUSINESS
December 30, 2021, 15:00 2 min reading
3D printed stem cells could save the spinal cord
3D printed stem cells could save the spinal cordSign in
Welcome!Log into your account
Your username
Your password
Have you forgotten your password?
Password recovery
Recover your password
Your email address
Home Science Medicine Stem clamps. ..
..
Injuries to the back and spinal cord are basically similar to damage to power lines - side, everything works fine, a damaged spinal cord will be the main cause of limb and nerve failure. Therefore, specialists and scientists have been struggling for years to develop the most effective and multifunctional type of treatment for various injuries of the back and spinal cord, some of which are very promising. For example, today neuroscientists from the University of Minnesota presented the results of their study on the development of this new type of treatment.
These are specially designed "bone pads" for various spinal regions, created using a powerful 3D printer - the basis of this technology is the use of silicon compounds and pluripotent stem cells. In other words, the scientists took several of these stem cells and turned them into biological spinal cord cells. Then, placing them on the indicated bone site, the specialists implanted this site with nerve cells in the affected area of several patients with various degrees of spinal cord injury.